What Is an eQMS?
An eQMS is an Electronic Quality Management System that helps businesses automate and streamline their quality management processes. With eQMS, quality teams can manage and track quality management-related activities, such as document control, change management, training management, supplier management, and many others, in a centralized and efficient manner.
Electronic Quality Management System (eQMS) also enables companies to monitor compliance with industry regulations and standards, and to identify and address issues proactively.
It is typically deployed on a cloud-based platform, accessible by all authorized users from anywhere, at any time.
The goal is simple: By automating quality management processes, businesses can improve product quality and ensure compliance while reducing costs and time to market.
We’ll expand on what eQMS exactly is, why companies use such a solution, how it works, and what makes a good eQMS solution in more detail below.
Furthermore, to help your search, we’ve compiled a list of the top 6 eQMS software solutions available to Life Science companies in 2023.
Streamline quality management and compliance processes with SimplerQMS’s eQMS software, tailored to the needs of the Life Science industry. Request a personalized demo today.
Jump to the specific topics covered in this article:
- What Is an eQMS?
- Why Do Companies Use an eQMS System?
- How Does an eQMS Work?
- What Makes a Good eQMS Software?
- The 6 Best eQMS Software Solutions for Life Sciences
- Frequently Asked Questions
What Is the Difference Between Electronic QMS and Enterprise QMS?
When people talk about an eQMS, they usually refer to an electronic quality management system software, and this is how we will use the term throughout this article.
However, an acronym for EQMS can also stand for “Enterprise Quality Management System”.
The latter is a broader term that encompasses an organization’s total approach to quality management, of which a typical eQMS software is just one part.
Since SimplerQMS provides broad QMS process support and follows the total QMS approach, we also consider ourselves an Enterprise Quality Management System provider.
Why Do Companies Use an eQMS System?
Organizations use electronic quality management systems for many reasons, but the overall goal is to improve quality, reduce costs, increase efficiency, and ensure compliance with applicable regulatory requirements.
In the context of life science organizations, operating in industries like medical devices, pharmaceuticals, biotech, and others, the requirements of a Quality Management System (QMS) are defined by regulations, standards, and guidelines such as:
- ISO 13485:2016
- FDA 21 CFR Part 820
- ICH Q10
- PIC/S GMP
- EU GMP
- EU MDR & EU IVDR
- And others
Here is an illustration of the main regulatory requirements that could apply to medical device and pharmaceutical organizations.
The goal of a QMS is to help organizations design and formalize a system that documents and controls their processes, so they can produce safe and effective products that meet customer requirements and regulatory standards.
Traditionally, Quality Management Systems have been implemented using paper-based methods, which are often inefficient and can lead to errors.
An eQMS system helps businesses overcome these challenges by automating quality management processes, making it easier to track and manage data, documents, and workflows.
A good way to determine if your organization would benefit from an eQMS is to ask yourself if you are struggling with any of the following challenges using a paper-based or hybrid QMS, which an eQMS system aims to solve:
- Limited traceability of documents and processes
- A high number of progress check meetings
- Lack of access to the paper archive
- The burdensome and challenging design transfer process
- Complicated risk management and risk analysis
- High risk of misfiled or lost documents
- Obsolete and out-of-sync records in circulation
- High risk of missing signatures
- Lack of accountability and visibility within teams
- Hard to keep up-to-date design documentation throughout the product lifecycle
- Ineffective and lengthy document authoring processes
- High costs connected to inefficiency, human error, and corrective measures
Do you find yourself facing one of these issues?
If so, an eQMS system might be the right solution for you.
SimplerQMS offers QMS software designed for life sciences, including organizations operating in the pharmaceutical, medical device, and other industries.
It is easy to use, pre-configured, fully validated, compliant out-of-the-box and can be implemented quickly.
To learn more about how SimplerQMS can help your organization, request a personalized demo.
How Does an eQMS Work?
An eQMS systems start by connecting all the quality-related processes and data in an organization in a single system. It provides a centralized repository for all documents and data, making it easy to manage your business processes and workflows, so you can streamline quality and compliance.
Controlled document workflows, QMS process support, electronic signatures, automated notifications and reminders, and real-time visibility into the progress of the tasks are some of the core features of a digital QMS.
In practice, an eQMS should make it easy to do the following:
- Create, edit, review, approve, and archive documents
- Sign off documents using electronic signatures
- Control document versioning as well as access, based on user roles and permissions
- Track and manage quality events, such as non-conformances, customer complaints, audit findings, and CAPAs
- Streamline other QMS processes, such as employee training, change control, design control, risk management, equipment calibration, supplier management, and others
- Generate reports, and analyze quality data to identify trends and areas for continuous improvement
- Plan, schedule, conduct, and document quality audits
- Help ensure compliance with quality standards
A good eQMS should work with the way your organization already operates and be flexible enough to adapt as your needs change.
There are many eQMS solutions out there, but not all of them are created equal.
But there is likely an eQMS system that’s the right fit for your organization. SimplerQMS is an example of an eQMS software purposefully built for life science organizations. The system supports core processes relevant to the industries, such as document control, design control, change control, supplier management, training management, audit management, and many more.
What Makes a Good eQMS Software?
Now, let’s take a look at some of the essential elements of modern QMS software.
QMS Process Support
First, you will need to make sure that the eQMS software you choose supports all of the quality processes that are relevant to your organization, such as:
- Document Control
- Change Control
- Training Management
- Supplier Management
- Design Control
- Risk Management
- Audit Management
- Non-Conformance Management
- CAPA Management
- Support for other processes, that might be relevant to your business
The number of features and services offered by different QMS software providers is vast and it’s easy to get overwhelmed. So, we created a free eQMS software comparison template, which you can use to compare different software vendors and find the solution that best meets your needs.
Compliance With FDA 21 CFR Part 11
If you’re in the life sciences industry, then you need to make sure that the eQMS software you choose is compliant with FDA 21 CFR Part 11.
This regulation sets forth the requirements for electronic records and electronic signatures and establishes the criteria under which they are considered trustworthy, reliable, and generally equivalent to paper records.
To learn more about this regulation and its requirements, check this article on the FDA 21 CFR Part 11 requirements.
Software Validation
Another important element to look for in eQMS software is whether it is validated.
If you’re in the life sciences industry, then you know that validation is a process that ensures that your computer systems and software meet FDA requirements and that they’re fit for their intended purpose.
Make sure to ask an eQMS vendor how validation is performed and who takes care of this task!
Ensuring that the system is continuously validated when the software changes can be a challenging, time-consuming, and expensive process.
One way to avoid this is to choose a fully-validated eQMS software that comes with a validation package and continuous validation services from the vendor, like SimplerQMS.
SimplerQMS does all the eQMS software validation for you – there are NO additional costs, resources, or time that should be allocated from your side.
To learn more about the topic of validation of an eQMS, check out this article on QMS software validation and when it is needed.
Avoid Digital Archives That Are Not Validated
If you currently have some type of a hybrid QMS system in place, with both paper and electronic records, then you might already be using some file server services like Dropbox, SharePoint, or Google Drive. The problem with these is that services are not necessarily meant for keeping and controlling digital records, especially for organizations in the life science industries.
They often lack the necessary certifications and validation.
Using non-validated software to handle your records and documentation is the quickest way toward non-conformances.
It’s tempting to use these alternatives instead of a purely paper-based QMS. They offer easier accessibility to your documentation but one of the main issues is that many services are not compliant with FDA CFR 21 Part 11 software requirements, which could lead to serious consequences for your business during audits and inspections.
So, if you’re using any of these file-sharing services in your life science organization for keeping and managing your quality documentation, we recommend that you switch to a software solution that is FDA 21 CFR Part 11 compliant.
Regulatory Compliance
The next thing you need to consider is whether an eQMS will help you assure regulatory compliance. So, you should check for compliance with the standards, regulations, and requirements applicable to your business, for example:
- ISO 13485:2016
- FDA 21 CFR Part 820
- FDA 21 CFR Part 11
- ICH Q10
- GxP
- GAMP 5
- EU Annex 11
- EU MDR and EU IVDR
- Etc.
These guidelines provide needed security and standardized regulatory requirements for Quality Management Systems.
To learn about Life Science Quality Management Systems and their requirements, explore the following articles:
- Pharmaceutical Quality Management System
- ICH Q10 Pharmaceutical Quality System
- Clinical Quality Management System
- Laboratory Quality Management System
- ISO 15189:2022 Compliant QMS
- Medical Device Quality Management System
- ISO 13485:2016 Compliant QMS
- 21 CFR Part 820 Quality System Regulation
Deployment Model
Lastly, you should also be mindful of how the eQMS will be deployed within your organization. In other words, you will need to find out what are the hosting options of the solution.
Non-cloud-based (on-premise) options will usually require you to set up your own servers and IT staff which might end up only increasing your costs.
On the other hand, cloud-based eQMS systems are often more affordable and easier to set up since they don’t require any special hardware and dedicated IT resources.
Such a deployment model allows users to access the system from any device and location. Cloud-based eQMS systems are often updated more frequently than on-premises solutions. So, you can be sure that you’re always using the latest software version and that your data is backed up and secure.
Plus, cloud-based solutions can be quickly deployed and scaled to meet the needs of a growing business, and are therefore often the preferred choice for smaller businesses and growing startups.
Apart from the factors we’ve already mentioned, you should also have in mind a few other considerations when choosing an eQMS, such as:
- How long does it usually take to implement the system?
- What support can I expect after the solution has been implemented?
- What kind of training material is available?
- Etc.
You can read more about this topic in our article about how to choose the right QMS software for your organization.
The 6 Best eQMS Software Solutions for Life Sciences
Below is a list of the top 6 eQMS solutions for Life Sciences on the market today with brief descriptions of which one is better for certain needs.
1. SimplerQMS
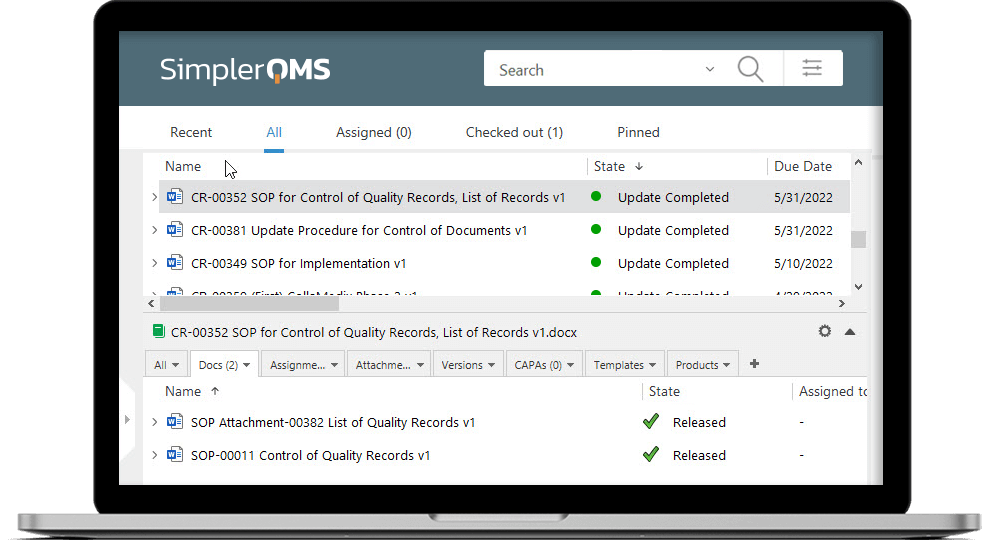
SimplerQMS (that’s us!) provides a fully validated, cloud-based eQMS software designed for Life Science industries.
Our solution offers automated workflows for a variety of QMS processes and native integration with Microsoft Office applications such as Word, Excel, and PowerPoint.
The software supports compliance with various Life Science requirements such as FDA 21 CFR Part 11, Annex 11, GxP, ICH Q10, FDA 21 CFR Part 210, 211, 820, ISO 13485:2016, ISO 9001:2015, EU MDR and IVDR, and more. Furthermore, we offer forms, templates, and procedures, developed explicitly for Life Science companies to make regulatory compliance just a little simpler.
SimplerQMS is validated according to GAMP5 and provides continuous re-validation. In other words, we handle all software validation processes on your behalf, eliminating any extra costs, or time allocation required for software validation on your end.
The annual subscription includes everything – implementation, training and onboarding, continuous validation, hosting, audit assistance, support, and all QMS modules. Moreover, you have the flexibility to select the modules you want to start with and add additional ones as needed, all without incurring any extra costs.
When evaluating SimplerQMS, keep in mind the following key considerations.
- SimplerQMS is fully validated and allows for quick configuration within a validated framework based on various regulatory requirements.
- It is best suited for companies that don’t want to spend time defining processes from scratch and avoid complex validation activities.
- Not suitable for those looking for a custom-developed eQMS.
- SimplerQMS is integrated with Microsoft Office, if an organization prefers not to use Microsoft Office for documentation, it may not be the best fit.
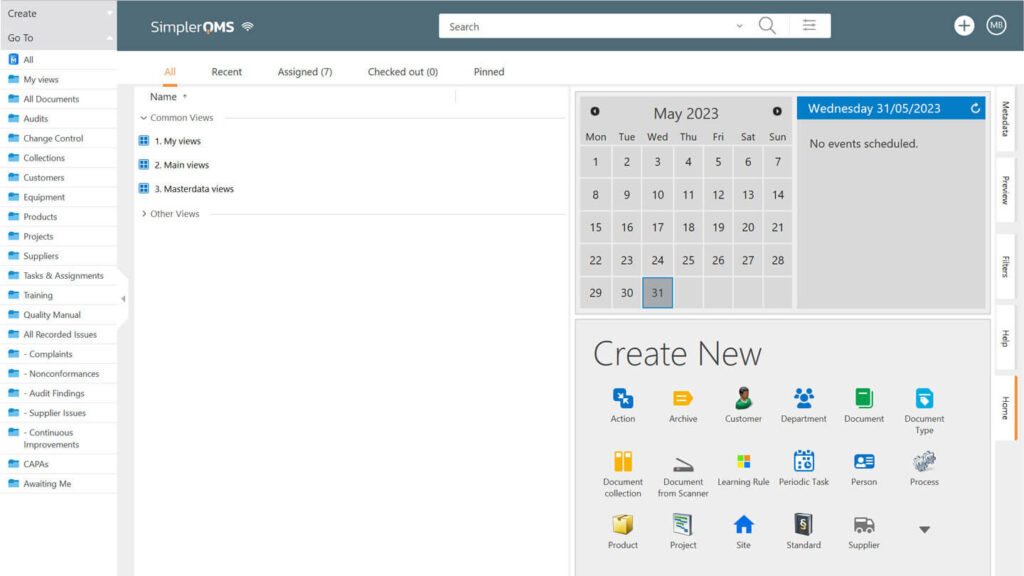
2. Qualio
Qualio is a cloud-based eQMS designed for startups, small to mid-size businesses, and Life Science companies in the scale phase.
Qualio helps streamline a variety of processes such as document control, training, non-conformances, complaints, CAPAs, supplier management, and others.
It aligns with various requirements in the Life Science industry and integrates with popular apps such as Google Suite, Asana, Jira, and Azure DevOps. Furthermore, Qualio provides access to a partner network of experts through Qualio+.
The software offers annual subscription plans based on plan type and number of users.
As you evaluate Qualio, keep in mind the following key considerations:
- Qualio uses its built-in editor, making it a suitable option for those who prefer not to use Microsoft Office for editing documents.
- Onboarding services must be purchased separately, and customers are responsible for performing PQ (Performance Qualification) validation.
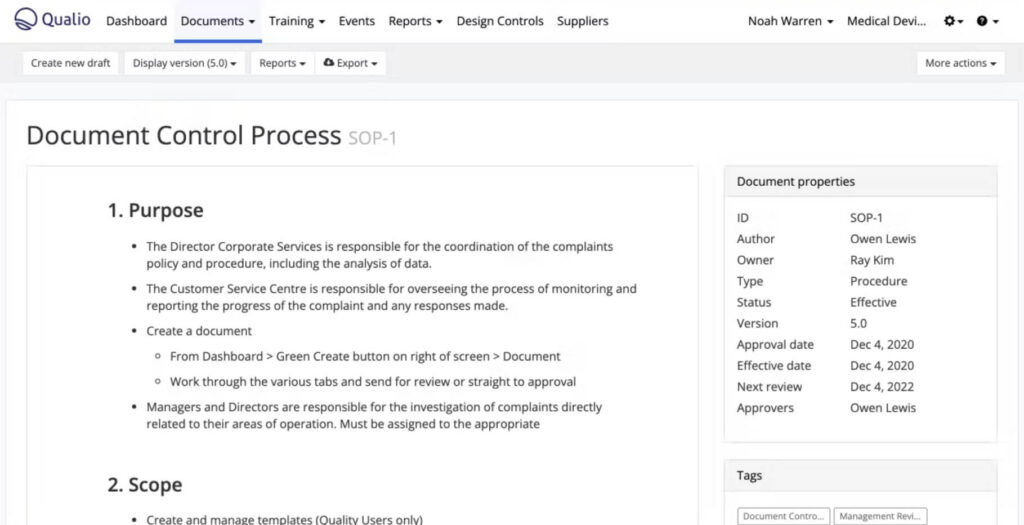
3. MasterControl
MasterControl is a renowned QMS solution, developed for life science and other highly-regulated industries.
It offers a comprehensive solution covering all aspects of quality and compliance, including document control, supplier, product development, manufacturing, audits, training, clinical trials, CAPA, regulatory risk management, and other processes.
The pricing for MasterControl’s QMS software is subscription-based and depends on factors such as the number of users, selected modules, and application functionality.
When evaluating MasterControl, keep in mind the following key considerations:
- MasterControl is an enterprise-level solution, primarily targeting large organizations with complex needs. It may not be the best fit for small to mid-sized organizations due to its extensive feature set and potentially higher price tag.
- MasterControl provides validation as part of the subscription package, including complete software validation documentation, and their patented Validation Excellence Tool (VxT)™. Continuous system validation is the customer’s responsibility after the initial validation is completed.
4. TrackWise
Sparta Systems provides two QMS software solutions, TrackWise and TrackWise Digital, designed to cater to the needs of life science and highly-regulated organizations.
TrackWise is an on-premise enterprise eQMS system that offers pre-configured industry best-practice workflows, which are adaptable and configurable to meet specific organizational needs.
TrackWise Digital is a cloud-based solution powered by the Salesforce platform, offering a flexible and scalable system with powerful reporting and analytics capabilities. TrackWise Digital enables companies to manage quality and compliance processes efficiently and effectively.
Pricing for either of the systems depends on the number of user licenses, selected quality processes, and implementation needs.
When evaluating the TrackWise solution, keep in mind the following considerations:
- Similar to MasterControl, TrackWise is an enterprise-level QMS software, which may not be the best fit for small to mid-sized companies due to its comprehensive features and higher price.
- TrackWise offers a high level of customization and complex options for organizations with specific requirements.
- Integration with other enterprise applications, such as CRM, ERP, LIMS, and MES systems, is possible, allowing for a more comprehensive and streamlined process.
5. Q-Pulse
Q-Pulse, an eQMS software solution from Ideagen, offers a comprehensive governance, risk, and compliance (GRC) solution for various industries, including life science, aerospace, defense, manufacturing, food and drink, and healthcare.
It covers a wide range of processes, such as document management, employee training, supplier management, quality control, safety, health, environment, audits, risk management, and more.
Q-Pulse helps automate and streamline processes while helping comply with regulations and standards like ISO 13485, ISO 9001, ISO 27001, ISO 50001, ISO 22000, ISO 29001, and others.
Similar to many other eQMS solutions, Q-Pulse follows an annual subscription pricing model.
Before choosing Q-Pulse, keep in mind the following key considerations:
- Q-Pulse targets a wide range of industries, allowing for the creation of highly specific workflows to meet specific needs. It is a suitable choice for those willing to take on the required validation and re-validation tasks that come with highly customizable software.
- Services such as implementation and validation (including IQ, OQ, and PQ) are not included in the subscription price and require a separate purchase, increasing the overall cost.
6. Greenlight Guru
Greenlight Guru is a cloud-based QMS software specifically designed for the medical device industry.
The platform offers a centralized system for managing all aspects of the product life cycle, from ideation to market launch.
Greenlight Guru integrates with Jira, an industry-leading issue and project tracking tool, to help MedTech companies accelerate product development while maintaining compliance.
Greenlight Guru offers three subscription plans based on the lifecycle stage, all billed annually with different features and price points.
As you evaluate Greenlight Guru, keep in mind the following key considerations:
- The built-in web editor allows for document editing within the interface. Those preferring an eQMS integrated with Microsoft Office may want to explore other options.
- The subscription price does not include implementation, data migration, and training services, which must be bought separately.
- Greenlight Guru handles the majority of the validation process, but the customer must perform the PQ validation.
You can refer to our complete comparison of these top eQMS software solutions for life sciences, which will help you find the ideal fit for your organization’s requirements.
Then, you can use our QMS software comparison template to compare your shortlisted eQMS software platforms against each other and choose the best one for your organization.
Frequently Asked Questions
What Does an eQMS System Actually Do?
An eQMS system streamlines and automates quality management processes like document control, change control, non-conformance, CAPA, audits, training, supplier management, and more.
Typically, an eQMS will store quality-related documents and data in a central repository, which can be accessed and updated by authorized users from anywhere in the world. Different departments within an organization can use an eQMS to collaborate on quality-related tasks, ensuring that everyone is on the same page and working towards the same goal.
What Are the Benefits of an eQMS?
There are many benefits to using an electronic quality management system, including improved communication and collaboration across the entire organization, automation, and streamlining of QMS processes, improved compliance with regulations and standards, better visibility into quality data, reduced costs, and improved efficiency.
When Is the Right Time to Adopt an eQMS?
Before investing in an eQMS solution, you should first have developed a QMS or be in the process of implementing one.
Once you have a QMS in place (or are in the process of developing it), you can start thinking about streamlining and automating your processes with an eQMS when:
- You’re using multiple spreadsheets, paper documents, or various software solutions to manage your quality processes
- You need to improve communication and collaboration within your quality department
- You need to automate and streamline your QMS processes, to improve efficiency, and reduce costs
- You need to improve compliance with regulations and standards
- You need better visibility into your quality data
- You need a solution that is scalable and flexible to accommodate your company’s growth
If you can relate to any of the above, then it’s probably time to start looking for an eQMS solution that will help you take your quality management to the next level.
How Much Does an eQMS Cost?
The price of electronic quality management systems ranges. There’s no one-size-fits-all answer. Therefore, many eQMS providers tailor their pricing models to the specific needs of each customer.
The main cost factors of an eQMS include:
- The number of users: Many eQMS providers charge based on the number of users that need access to the system. The price per user usually decreases as the number of users increases.
- The number of QMS processes, or modules, you need: Some eQMS solutions offer a variety of modules that support different QMS processes. Such vendors usually charge based on which modules you need.
While the factors of eQMS pricing can be complex, the good news is that the barriers to entry for eQMS software have never been lower.
The main reason we offer SimplerQMS as an all-in-one eQMS software solution for one subscription price (depending on the number of user licenses) is to ensure that quality management is accessible and affordable for businesses of all sizes.
This means that all QMS modules, implementation, training, ongoing support, updates, cloud-based hosting, and more are included in the QMS software pricing. There are no hidden costs or unexpected fees, which makes it much easier to budget for an eQMS solution.
How Do You Validate an eQMS?
You validate an eQMS by executing test scripts and ensuring it is working as intended. The entire process is documented according to the process for Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
By choosing SimplerQMS as your eQMS vendor your organization won’t need to validate the system to comply with ISO and FDA regulations concerning system validation. We provide the system in a fully-validated state and perform continuous re-validation.
Final Thoughts
An electronic quality management system is a powerful tool that can help organizations to improve quality and compliance, increase efficiency, and reduce costs.
It outperforms traditional paper-based quality management systems in many ways and is quickly becoming the new standard for quality management across all industries.
If you’re looking for a way to take your quality management to the next level, an eQMS may be the right solution for you.
SimplerQMS offers a cloud-based eQMS software solution designed specifically for the life science industries. Request a demo today to learn more.