Drive Quality Up the Agenda
Help everyone in your organization seamlessly engage with your quality processes.
TRUSTED BY
























challenge
Inefficient QA Processes
Managing your Quality Assurance (QA) documentation in a manual system takes time and increases the risk of human errors.
Therefore, a system that digitizes and automates document control and other processes, is so useful.
solution
Automated Document Control Workflows
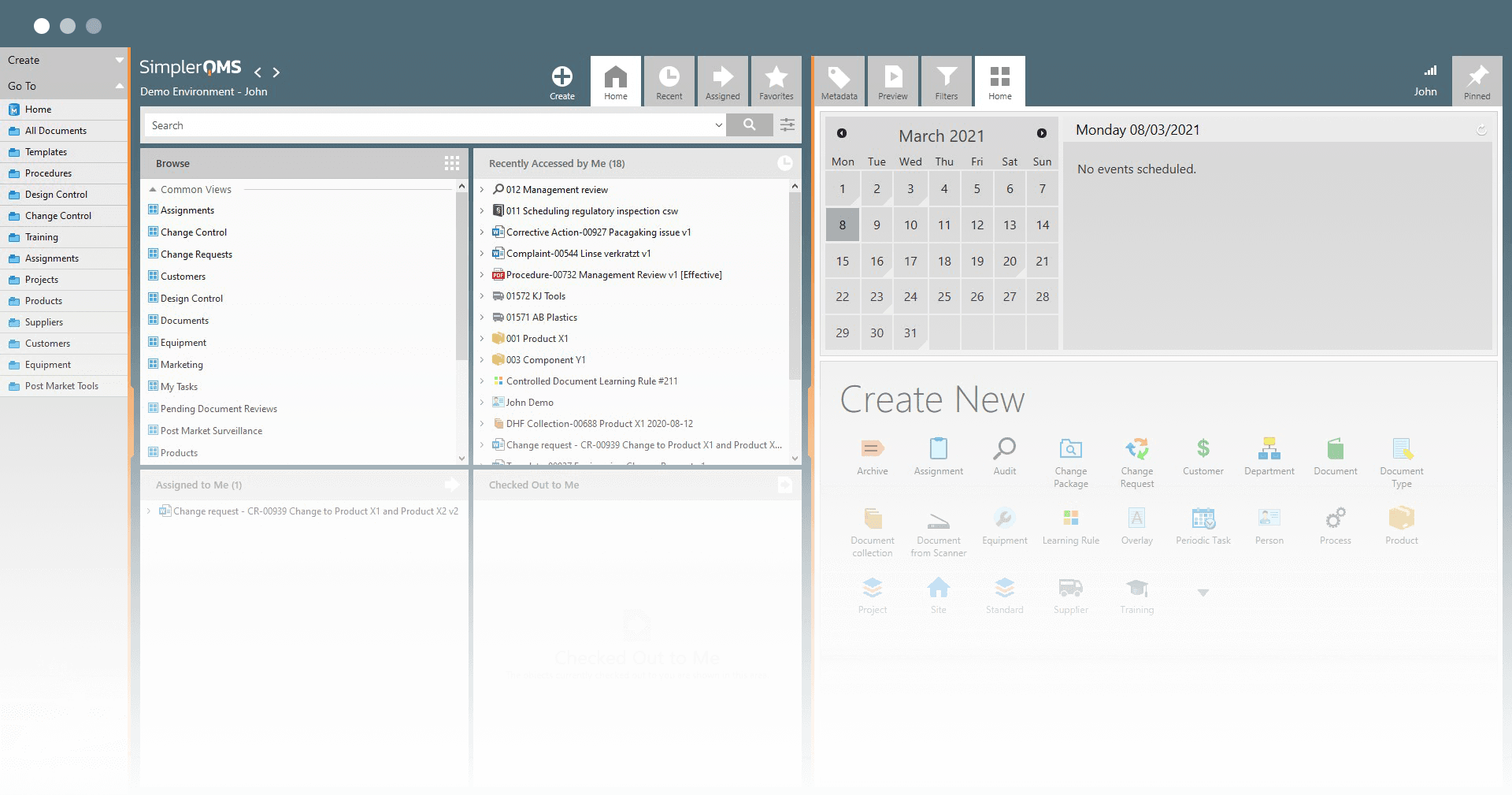
SimplerQMS allows you to work on your documents in a familiar Microsoft Office interface and save documents with one click.
No need to download, edit, and upload the document every time you make a change. Documents are automatically named, numbered, and versioned.
This saves you time, improves your documentation structure, and provides a better overview.
solution
Fully Validated QMS Software Solution
Computer System Validation is a requirement for Life Science companies that store and manage quality records digitally.
SimplerQMS provides a fully validated eQMS solution that is validated according to GAMP5 and undergoes revalidation for each new version or standard update.
SimplerQMS complies with requirements regarding the validation of systems used for QMS such as 21 CFR Part 11, and 820, EU GMP Annex 11, and ISO 13485:2016.
This means that your QA department does not have to spend any time or resources on software validation. We do all the software validation for you, eliminating any additional expenses, resources, or time commitments on your part.
challenge
Lack of Employee Ownership
Quality Assurance (QA) departments typically struggle with participation from the rest of the organization with quality-related processes.
Tasks tend to be completed with a delay, which can cause issues with regulatory compliance. This is why automated workflows that notify users every time they need to act are so important.
solution
Streamlined Quality Assurance (QA) Processes
With SimplerQMS, you can automate data collection, routing, notifications, follow-ups, approvals, and escalation of activities.
Automated workflows ensure that the relevant persons are notified when they are assigned a specific task. You can easily track everyone’s performance towards their due dates through customizable Dashboards and Notifications.
challenge
Weak Culture of Compliance
As a Quality Assurance (QA) leader, you must have visibility of the performance of quality systems in your organization. This allows you to identify a non-compliant behavior, correct it before it escalates, this way being more prepared for audits.
solution
Connected Data Across All Quality Processes
With SimplerQMS, you can easily create hyperlinks to products, components, suppliers, customers, and equipment from any quality event. Upload and link any document, file, record, and even an email to audits, risks, or CAPAs, as evidence.
Integrate different quality processes and store everything in a single location. This way you will gain better visibility of non-conformances and will be able to determine root causes more easily.
solution
Full Regulatory Compliance
SimplerQMS software solution helps you stay compliant by automatically creating and storing a time-stamped audit trail of every document change. Our built-in 21 CFR Part 11 compliant electronic signatures and automatic workflows allow you to forget about the manual circulation of records and chasing wet signatures.
Furthermore, SimplerQMS offers a solution that supports compliance with 21 CFR Part 11, EU GMP Annex 11, GxP, ISO 13485:2016, ICH Q10, EU MDR and IVDR, FDA 21 CFR Part 201, 211 and 820, and many other Life Science requirements.
modules
The SimplerQMS Modules Cover All Your Processes
No matter if your life science organization is a start-up or a later-stage company, our integrated modules cover your needs. All of the modules are included in the SimplerQMS subscription.
Training Management
Save time with automated training activities, learning overview, reminders, and generation of training certificates.
CAPA Management
Identify, uncover, resolve, and report all the preventative actions and corrective actions (CAPAs) seamlessly.
Complaint Management
Reduce the associated risks and resolve issues quickly by optimizing complaint management processes.
Change Management
Recognize and manage all changes accordingly to ensure compliance and structure within your organization’s QMS.
Design Control
Manage all the necessary processes related to product design and meet design control requirements with ease.
Document Control
Automate and standardize your document control activities with ease.
Risk Management
Consolidate risk and handle your risk management file in a well-organized and structured manner.
Supplier Management
Simplify supplier-related activities and handle your supplier documentation following the standards.
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.