Pharmaceutical Quality Management System (QMS) is a set of procedures and practices contributing to product quality.
The QMS must reflect the relevant regulatory requirements applicable to the industry and company. In the pharmaceutical industry, some of the important quality standards and guidelines are ISO 9001:2015 and ICH Q10.
This article will cover what quality is in the pharmaceutical industry, the Pharmaceutical Quality Management System, some pharmaceutical QMS elements and requirements, and the role of quality management software.
SimplerQMS offers fully validated, cloud-based pharmaceutical QMS software. Our system integrates all QMS modules to simplify document management, change control, training management, audit management, deviation management, CAPA management, supplier management, and other processes.
If you are interested in discovering how SimplerQMS can improve your company’s quality processes, schedule a personalized demo and talk to our experts.
Here is everything you will learn in this article:
- Definition of Quality in the Pharmaceutical Industry
- What is Pharmaceutical Quality Management System (QMS)?
- Applicable Pharmaceutical QMS Regulations and Standards
- Elements of Pharmaceutical Quality Management System
- Role of Pharmaceutical Quality Management Software
Definition of Quality in the Pharmaceutical Industry
Quality in the pharmaceutical industry refers to the degree to which a drug substance or product meets its intended use and fulfills its inherent properties. This definition includes essential attributes like the drug’s identity, strength, and purity.
The quality of a drug is critical for patient safety and effective treatment. If a drug is contaminated or not pure, it could cause harm to patients. Similarly, if a drug is not potent enough, it may not be effective in treating the condition for which it was intended.
Before pharmaceutical products are approved for human use, they must comply with regulatory requirements. In this regard, a Pharmaceutical Quality System (PQS) includes all processes of a system that helps ensure quality in the pharmaceutical product lifecycle.
What is Pharmaceutical Quality Management System (QMS)?
Pharmaceutical Quality Management System (QMS) is a comprehensive collection of policies, processes, and procedures designed to ensure and maintain uniform and high quality in the production of pharmaceutical products.
The QMS must reflect the specific needs of the pharmaceutical company and applicable regulatory requirements.
A robust Pharmaceutical Quality System (PQS) that reflects the applicable requirements can also help pharmaceutical companies to mitigate risks, improve customer satisfaction, and streamline quality processes.
The pharmaceutical quality management system includes several processes, such as:
- Document management
- Change control
- Training management
- Audit management
- CAPA management
- Deviation management
- And others
The quality system uses monitoring methods such as Quality Assurance to prevent quality deviations and emphasizes quality system documentation to record all problems and their solutions.
You can read our guide on what Quality Management System (QMS) is to learn more.
SimplerQMS provides QMS software for the pharmaceutical industry to help companies streamline all quality processes in one solution. You can easily manage documents, changes, training, audits, deviations, CAPAs, suppliers, equipment, and much more.
Applicable Pharmaceutical QMS Regulations and Standards
Establishing and working according to the Quality Management System (QMS) is a requirement in the pharmaceutical industry. To ensure uniform and high-quality products, companies need to implement a QMS that reflects the requirements applicable to them.
The company is responsible for complying with the relevant regulations and standards, which depend on many factors, including geographical location, product type, and target market.
Some of the most common standards and regulations applicable to the pharmaceutical QMS are described below.
International Organization for Standardization (ISO)
The International Organization for Standardization (ISO) is an international body developing standards for various industries, including Pharmaceuticals. The current standard for Quality Management Systems (QMS) is ISO 9001:2015, and previously it was ISO 9001:2005.
Some pharma companies use this standard to improve performance and ensure that their QMS complies with the requirements for quality.
Pharmaceutical Inspection Co-operation Scheme (PIC/S)
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) is a non-binding, informal collaborative arrangement between international Regulatory Authorities in the field of Good Manufacturing Practice (GMP) of medicinal products for human or veterinary use.
The PIC/S publishes guidance documents such as the PIC/S GMP Guides that specify various requirements for pharmaceutical companies, including a pharmaceutical quality management system. The requirements in the PIC/S GMP are equivalent to the EudraLex Volume 4 GMP in terms of good manufacturing practice guidelines.
Pharmaceutical companies use PIC/S GMP guidelines to provide a framework for quality management systems, manufacturing processes, and control systems to ensure products are safe, effective, and of uniform and high quality.
The guides cover various aspects of GMP compliance, such as:
- Premises and equipment
- Personnel
- Documentation
- Quality control
- Complaints and product recall
- Self-inspection
ICH Guideline Q10
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) is a non-profit association under Swiss law. It aims to achieve greater pharmaceutical requirements harmonization worldwide.
The combination of the letter Q and specific numbers, such as Q8, Q9, etc., designates the Quality guidelines.
The Q10 guideline is for the Pharmaceutical Quality System.
It provides a consolidated structure for the quality procedures based on established standards, regulations, and guidelines, including:
- ISO
- cGMP
- ICH Q8 Pharmaceutical Development
- ICH Q9 Quality Risk Management
We recommend reading our article on the ICH Q10 Pharmaceutical Quality System if you want to know more about this guideline. You will also learn about the role of an electronic QMS (eQMS) in helping achieve compliance with ICH Q10.
Current Good Manufacturing Practice (cGMP)
The current Good Manufacturing Practices (cGMP) are regulations enforced by the Food and Drug Administration (FDA), the US federal agency responsible for the safe production of drugs.
The product must be safe for human use, with quantity, quality, and purpose taken into consideration. The output must be free from contamination and prevent the mix-up of one product with another.
You can check out our article for a brief introduction to cGMP regulations.
The FDA inspects drug manufacturers for cGMP compliance. Non-compliance with cGMP regulation may lead to product recalls when deemed necessary upon investigation. Failure to follow the subsequent FDA directions can result in seizure, fines, and jail time.
Feel free to read our article about how GMP compliance is reflected in QMS to learn more about GMP-compliant quality management systems.
FDA 21 CFR Part 210
The 21 CFR Part 210 regulation specifies the minimum cGMP requirements for drug manufacturing, processing, packing, and storage to ensure product safety, quality, and purity.
Compliance with this regulation is mandatory for pharmaceutical companies marketing and selling their products in the United States.
FDA 21 CFR Part 211
21 CFR Part 211 specifies the cGMP requirements for finished Pharmaceuticals.
This regulation covers the following areas:
- Quality control
- Qualification and skills of the personnel
- Buildings and facilities
- Ventilation and air filtration system
- Equipment cleaning and maintenance
- Product labels
- Warehouse requirements.
21 CFR Part 11
The 21 CFR Part 11 regulates how electronic records are developed, maintained, and stored. It also describes how the relevant supervisors authorize these documents using electronic signatures.
If you want to learn more about this regulation, read our article on 21 CFR Part 11 requirements.
EU GMP Annex 11
The EU GMP Annex 11 provides good manufacturing guidelines for computerized systems.
When replacing a manual process with a computerized system, it is important to ensure that product quality, process control, and quality assurance are not compromised. Moreover, the overall risk of the process should not increase.
It also includes guidelines for the following topics:
- Data Storage
- Security
- Electronic Signature
- Batch release
- Personnel
- Risk Management
ISPE GAMP5
The Good Automated Manufacturing Practice 5 (GAMP5) is a guideline developed by the International Society for Pharmaceutical Engineering (ISPE) for computerized systems.
The goal of GAMP is to provide a cost-effective framework of best practices that ensures computerized systems are effective, of high quality, and compliant with applicable regulations while also being suitable for their intended use.
SimplerQMS provides an eQMS solution tailored to the pharmaceutical industry. The system supports compliance with ISO, ICH, cGMP, EU, and PIC/S GMP requirements. It is also validated in accordance with ISPE GAMP5 and is re-validated upon new version releases or standard updates, allowing you to save time and focus on value-adding activities.
Our software complies with FDA 21 CFR Part 11 and EU GMP Annex 11, ensuring compliance with electronic records, electronic signatures, and computerized system manufacturing guidelines.
Elements of Pharmaceutical Quality Management System
A Pharmaceutical Quality System comprises numerous elements and QMS processes. There is no “fixed” implementation scheme. The drug manufacturer can integrate these processes as per the product and intended market requirements.
This article will also provide examples of how QMS software can simplify and optimize processes.
NOTE
In this article, we will provide a brief overview of certain elements of the Pharmaceutical Quality System outlined in the ICH Q10 guideline related to the product lifecycle, as well as additional elements discussed in the FDA regulations and PIC/S GMP guidelines. It is important to note that companies should always refer to the original requirements for complete information.
Some of the elements of the product lifecycle in ICH Q10 are the following:
- Process performance and product quality monitoring system
- Corrective action and preventive action (CAPA) system
- Change management system
- Management review of process performance and product quality
Process Performance and Product Quality Monitoring System
The process performance and product monitoring system is an integral part of a company’s efforts to deliver a uniform and high-quality product. It shows the company’s commitment to sustained resources and control.
Process performance and product quality monitoring are applied to four stages of the product lifecycle:
- Pharmaceutical development: The knowledge gained about the process and product during development and monitoring can be utilized to create a manufacturing control plan.
- Technology transfer: During scale-up activities, monitoring can indicate process performance and successful integration into manufacturing.
- Commercial manufacturing: A robust system must be implemented for monitoring process performance and product quality to ensure consistent control and identify opportunities for improvement.
- Product discontinuation: Stability testing and monitoring should continue to the completion of the studies, even after manufacturing ceases.
Risk assessment techniques can be used to detect shortcomings in process stages. Specialized tools and feedback can also be used to analyze given parameters.
Production personnel and the Quality Department check the inspection system randomly to identify faults or malfunctions.
Luckily, a tool like Deviation Management Software by SimplerQMS automates data collection, routing, and notification of overdue activities. As a result, monitoring your product and process performance and safety becomes much easier.
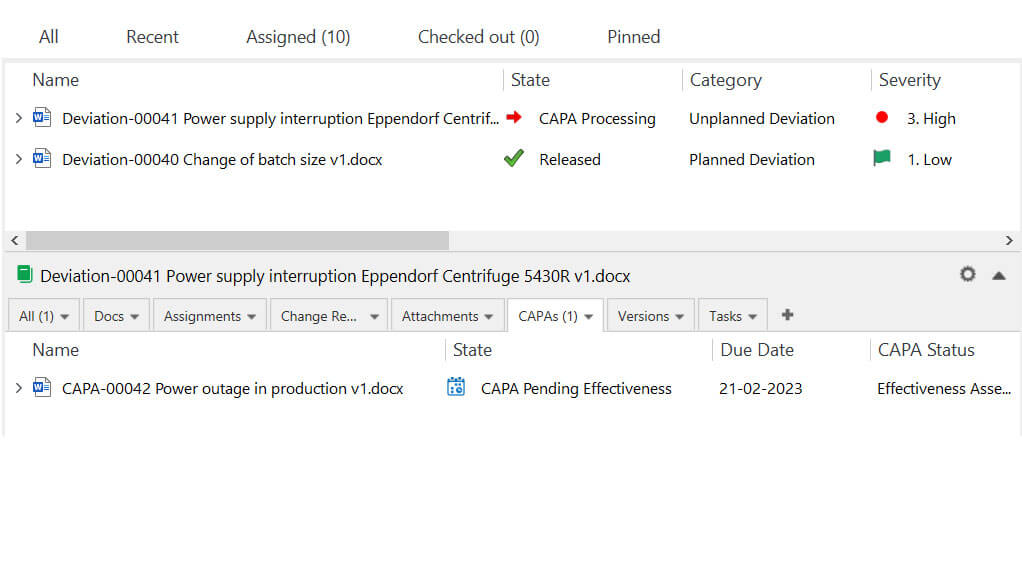
Corrective Action and Preventive Action (CAPA) System
Corrective Action and Preventive Action (CAPA) is a system for identifying the causes of issues and preventing them from recurring in the future.
Complaints, deviations, audit findings, and other issues can be escalated to a CAPA. Once the decision regarding the escalation to a CAPA has been made, the CAPA form is filled.
The CAPA structure and CAPA form can be different in each company, but mainly it consists of the following information:
- The department where process deficiency is found
- Issue detail and its implications
- The impact of the error on the process or product
- Corrective action to resolve the problem
- Preventive action to prevent the recurrence of the issue
- Due date to implement the corrective and preventive actions
- Next review date to assess the effectiveness of corrective action
The typical CAPA process follows the steps illustrated below.
After necessary corrective and preventive actions are applied, the CAPA is sent again to the responsible persons for approval. The CAPA is closed and documented as part of the records upon approval.
The CAPA process is applied in different product stages, including pharmaceutical development, technology transfer, commercial manufacturing, and product discontinuation, with a focus on feedback, feedforward, continuous improvement, and periodic checks of the CAPA effectiveness.
The process presented in the example above can easily be automated with CAPA Management Software. It automates data collection, routing, follow-ups, notifications, and escalation of overdue activities allowing you to manage corrective and preventive actions more effectively.
To better understand how this process works, you can read our article about what CAPA is in the pharmaceutical industry.
Change Management System
Pharmaceutical companies must have a change control management system to control and manage all changes made to documents and processes.
The change management system can be applied to the product lifecycle in different stages:
- Pharmaceutical Development: Changes during the development process should be documented, and the change management process’s formality should match the pharmaceutical development stage.
- Technology Transfer: The process changes made during technology transfer activities should be managed and documented.
- Commercial Manufacturing: The quality unit’s supervision should ensure that there is adequate scientific analysis and evaluation based on risk for a change.
- Product Discontinuation: Any modifications made after discontinuing the product should still undergo a suitable change management process.
Fortunately, Change Management Software by SimplerQMS makes the change management process much easier.
It allows you to automatically document the change process, create and delegate specific assignments that will be electronically signed when approved, route changes for review and approval, set up reminders, and more.
Management Review of Process Performance and Product Quality
Management review is a comprehensive evaluation of the entire quality management system’s efficiency. This process includes assessing the product, documents, processes, and procedures.
The goal of the management review is to assess whether the QMS is functioning as intended by the company and identify areas for improvement.
The management review could include the following:
- The commitment made to the quality
- Customer complaints
- Nonconformances
- CAPA review
- Audit findings
- Process performance
- Any pending matter or follow-up from previous management review
Management review is essential throughout the whole product lifecycle stages.
During pharmaceutical development, management review should evaluate the adequacy of the product and process design. Meanwhile, during technology transfer, management review should ensure that the product and process design are suitable for commercial-scale manufacturing.
For commercial manufacturing, a structured system for management review must be followed to facilitate continuous improvement. Additionally, management review during product discontinuation should encompass various aspects, including product stability and quality complaints.
Overall, management review is crucial for ensuring the quality and consistency of pharmaceutical products, from development to discontinuation.
SimplerQMS helps in management review by providing a structured approach for evaluating the performance of a pharmaceutical company’s processes and products.
Our system collects and stores data, which can then be used to create pharmaceutical quality KPI reports and trending of key quality metrics.
You can also export all information to create complex data visualizations.
Other processes in FDA regulations, EU GMP, and PIC/S GMP guides outside the product lifecycle that we would like to focus on in this article are:
- Employee training management
- Equipment calibration and maintenance
- Supplier management
- Audit management
Employee Training Management
The pharmaceutical company should provide training for all the personnel whose activities could affect the quality of the product.
Personnel should receive training on the theory and practice of the Pharmaceutical Quality System and Good Manufacturing Practice, as well as specific training tailored to their job duties. Ongoing training should be provided and periodically evaluated.
Having control of employee training enable you to identify skill gaps, facilitate learning activities, and maintain detailed training records that meet compliance requirements.
SimplerQMS training management module helps companies with a range of features designed to support the training process, including assessments of training effectiveness, tracking of training progress, automated assigning of training activities, automated email notifications, and more.
Equipment Calibration and Maintenance
Equipment calibration and maintenance are critical to ensuring the quality and safety of the products produced in the pharmaceutical industry.
The purpose is to ensure that the equipment used in the manufacturing process is functioning properly and providing accurate and consistent results.
Written procedures are required for calibration, cleaning, and maintenance of equipment used in drug product manufacture. Implementing and following procedures are essential to prevent malfunctions or contamination that could affect the drug product’s safety, identity, strength, quality, or purity.
Using equipment management software by SimplerQMS, pharmaceutical companies can optimize their equipment control processes and easily create maintenance and calibration plans.
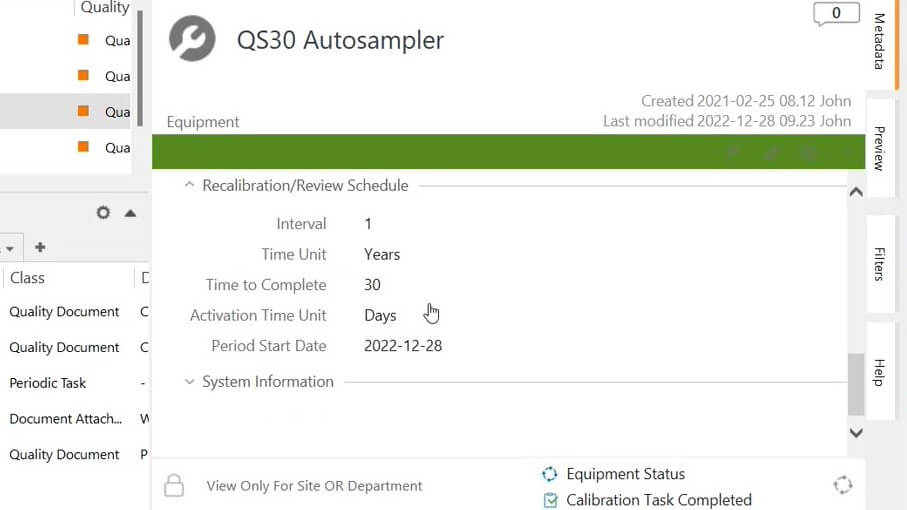
It’s possible to develop a list of equipment sorted into categories and to plan and schedule calibration and maintenance activities. You can easily track equipment status, overview ready-to-use equipment, automate notifications for upcoming tasks, as well as link products to equipment.
Supplier Management
Effective supplier management can help ensure a reliable supply chain, minimize the risk of product quality issues, and help pharmaceutical companies comply with regulatory requirements to control materials used in the manufacturing process.
Companies need to have a documented system for selecting, qualifying, and monitoring suppliers of raw materials, packaging materials, and other critical product quality components.
The supplier management process should include a risk-based approach that considers the potential impact on the quality and safety of the finished product.
SimplerQMS offers comprehensive supplier management capabilities to help streamline all supplier-related activities, from qualifying suppliers to maintaining Approved Supplier Lists (ASL), to monitor their subsequent performance.
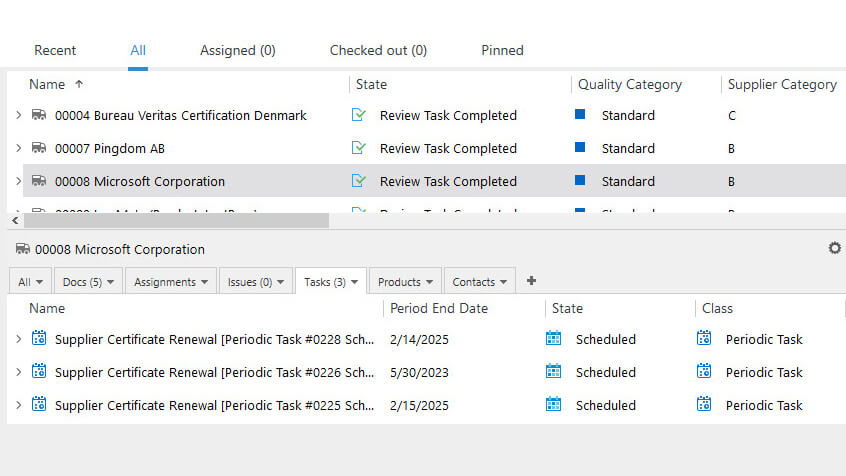
Audit Management
Self-inspection is an important component of the GMP requirements. Companies should conduct internal audits to monitor the implementation and compliance with GMP principles and to propose necessary corrective measures.
Internal audits should be conducted in a detailed way by assigned competent personnel from the company.
Additionally, independent audits by external experts can also be useful in verifying compliance with GMP requirements.
The audit plan should cover all manufacturing and quality control operations aspects, including facilities, equipment, personnel, materials, production, testing, and documentation. It should also cover any relevant supplier-related processes.
SimplerQMS also provides tools to effectively manage internal audits by proactively identifying and addressing potential quality issues.
Using our audit management module, companies can schedule and plan supplier, regulatory, external, and internal audits, assign responsibilities, and track progress. The system also facilitates maintaining a record of audit findings and actions taken.
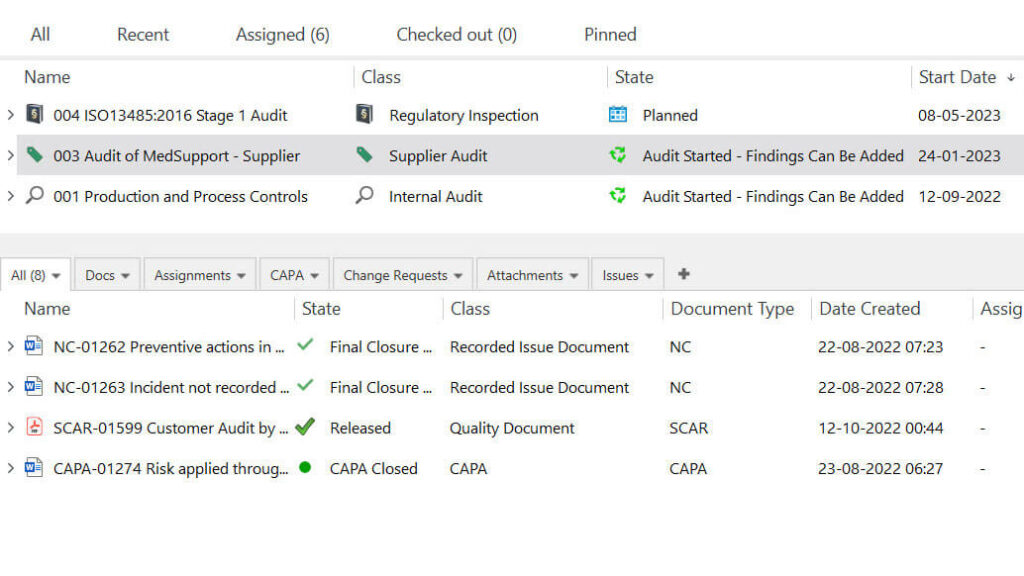
Role of Pharmaceutical Quality Management Software
Companies can manage their QMS using manual solutions if the required resources to handle the manual paperwork are available.
However, pharmaceutical quality systems can be complex due to the multiple interlinked processes involved and the high level of documentation required.
The digital revolution in drug manufacturing industries has paved the way for more production with less cost and time. Digital tools can take care of routine processes without compromising product quality, allowing company owners to focus more on innovation and exploring new avenues.
The pharmaceutical industry is already used to digital tools such as Computerized Maintenance Management Systems (CMMS) and Warehouse Management Systems (WMS). These tools effectively streamline processes with a proven track record.
Recently, an increasing number of companies have been opting to implement eQMS. A comprehensive eQMS provides several benefits, including improved workflows, reduced time and costs, cloud-based document storage, automated notifications, and more.
At SimplerQMS, we provide fully validated pharmaceutical quality management software.
Our eQMS not only simplifies employee training and document management but also streamlines change control, CAPA management, deviation management, audits, and many other processes.
Furthermore, to help companies build comprehensive documents, we offer a complimentary template package based on Life Science requirements.
Our software complies with all the relevant regulations and standards, such as FDA 21 CFR Part 11, which sets the guidelines for electronic signatures and electronic records, and EU GMP Annex 11, which provides good manufacturing guidelines for computerized systems.
Additionally, you don’t have to worry about computer system validation. SimplerQMS platform is fully validated according to ISPE GAMP5 and is re-validated upon the creation of a new version or upon applying standard updates.
If you are uncertain about the potential value of investing in an eQMS for your company, we recommend that you explore our eQMS Business Case template – available for download below.
This tool can assist you in evaluating the value of an eQMS for your company and includes the essential material for presenting your findings to the management.
Final Thoughts
Many regulatory requirements regulate the pharmaceutical industry.
The applicable regulatory requirements may vary depending on product type and intended market. Some pharmaceutical requirements include the ISO 9001:2015, ICH Q10, EU and PIC/S GMP, FDA 21 CFR Part 210 and 211.
One of the requirements is to implement a pharmaceutical quality management system and adhere to the processes outlined in it. The QMS enables companies to streamline their quality processes and helps ensure compliance to manufacture safe, efficient, and reliable products.
A digital tool like pharmaceutical QMS software from SimplerQMS helps pharmaceutical companies focus more on problem-solving than routine documentation processes. With our eQMS software, companies can streamline QMS processes to save time and help achieve compliance easier.
Book a free demo to see SimplerQMS Quality Management Software in action and learn how it can help your organization achieve better quality management processes.