The ICH Q10 Pharmaceutical Quality System (PQS) is a guideline that outlines a model for an effective quality management system for the pharmaceutical industry.
ICH Q10 provides a comprehensive model based on the International Standards Organization (ISO) quality concepts. It also incorporates relevant Good Manufacturing Practice (GMP) regulations.
The purpose of the guideline is to ensure the establishment of robust quality systems that support the consistent production of safe and effective pharmaceutical products.
This article provides an overview of the ICH Q10 Pharmaceutical Quality System, highlighting its importance and Quality Management System model. We also explore the role of eQMS software in implementing and maintaining the QMS that aligns with ICH Q10 principles.
There is a growing trend of Life Science companies adopting digital QMS solutions. These solutions provide an efficient and streamlined approach to managing quality processes.
SimplerQMS offers QMS software suited for pharmaceutical companies seeking to implement the ICH Q10 Quality Management System model. Schedule a personalized demo and talk to our experts to discover how SimplerQMS can streamline your company’s quality processes and help you work more efficiently.
Here is what we will discuss in this article:
- What Is the ICH Q10 Quality Management System?
- Why Is ICH Q10 Quality Management System Important?
- ICH Q10 Pharmaceutical Quality System Model
- Role of Pharmaceutical eQMS Software
- Frequently Asked Questions
What Is the ICH Q10 Quality Management System?
The ICH Q10 Quality Management System is a model for a pharmaceutical quality system that can be implemented throughout the different stages of a product lifecycle.
It is established by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH).
ICH Q10 applies to systems supporting the production of drug substances and drug products, including biotechnology and biological products, throughout their entire lifecycle.
These systems control essential processes such as formulation development, drug manufacturing, retention of documentation, quality control, change management system, CAPA system, and more.
Life Science companies adopting ICH Q10 are among others pharmaceuticals and biotechnological manufacturers, and contract research organizations (CROs).
ICH Q10 is part of the tripartite guideline that includes ICH Q8 and ICH Q9.
These guidelines collectively cover different aspects of product development and manufacturing in the pharmaceutical industry.
Let’s explore what each guideline, ICH Q8, Q9, and Q10, covers:
- ICH Q8 (Pharmaceutical Development): This guideline provides guidance on applying scientific approaches and quality risk management during the development and manufacturing of pharmaceutical products.
- ICH Q9 (Quality Risk Management): This guideline focuses on systematic approaches to quality risk management, ensuring that companies address potential risks effectively in their operations.
- ICH Q10 (Pharmaceutical Quality System): This guideline provides a model for the quality management system in the pharmaceutical industry, ensuring consistent quality throughout the product lifecycle.
ICH Q10 is built upon the foundation of regional Good Manufacturing Practice (GMP) requirements and is intended to be used in conjunction with them.
SimplerQMS provides efficient QMS software for pharmaceutical companies with integrated quality management processes. It helps ensure compliance with the ICH Q10 guideline, GMP requirements, FDA regulations, ISO standards, and more.
Why Is ICH Q10 Quality Management System Important?
The ICH Q10 Quality Management System is important because it provides a structured framework for pharmaceutical companies to establish and maintain a culture of quality.
This framework helps companies to ensure compliance with regulatory requirements, mitigate risks, and continually improve their processes and products.
ICH Q10 Pharmaceutical Quality System Model
ICH Q10 Pharmaceutical Quality System Model provides guidelines and principles for establishing, implementing, and maintaining a quality management system in the pharmaceutical industry and consists of four core areas.
- Pharmaceutical Quality System: Encompasses the overall quality system, including objectives, quality manual, quality risk management, and knowledge management.
- Management Responsibility: Focuses on the role of management in establishing quality objectives, allocating resources, and ensuring appropriate communication within the company.
- Continual Improvement of Process Performance and Product Quality: Emphasizes the need for ongoing improvement in process performance and product quality through data analysis, corrective and preventive actions, and customer feedback.
- Continual Improvement of the Pharmaceutical Quality System: Highlights the importance of continually improving the quality system itself through the use of management review, revisions to quality policy, and assessment of system performance.
NOTE
Please be aware that the information provided here is for educational purposes only. Companies must always refer to the official information in the ICH Q10 to ensure compliance.
This section will discuss the contents of the guideline model, represented in the ICH Q10 Pharmaceutical Quality System Diagram below.
At the top of the diagram, the Pharmaceutical Quality System (PQS) covers the entire product lifecycle stages, including development, technology transfer, manufacturing, and discontinuation. It complements GMP requirements specific to each country and also applies to investigational products.
The management responsibilities should be present across all stages of the product lifecycle, just like the PQS elements that form one of the pillars of the model.
The lower horizontal bars represent the enablers – knowledge management and quality risk management. These enablers support the PQS goals of product realization, state of control, and continual improvement.
In the following sections, we will further explore the ICH Q10 Pharmaceutical Quality System diagram to understand its key parts and their relations better.
ICH Q10 Lifecycle Stage Goals
The product lifecycle stage goals include the following technical activities for new and existing products according to the ICH Q10 section 3.1.
- Pharmaceutical Development
- Technology Transfer
- Commercial Manufacturing
- Product Discontinuation
Pharmaceutical Development
In the pharmaceutical development phase, the goal is to design products and plan the steps for the manufacturing process.
The company should focus on maintaining consistent process performance and meeting the needs of patients, healthcare professionals, regulatory authorities, and internal customers.
The technical activities conducted in this phase are:
- Developing drug substances
- Developing formulations that include both container and closure systems
- Developing analytic methods
- Developing manufacturing processes and plans for the scale-up
- Manufacture of investigational products
ICH Q10 applies to the development and manufacture of all pharmaceutical products. This also applies to pharmaceutical products still in the development stage and have not yet been approved for marketing, the so-called investigational products.
Technology Transfer
The technology transfer phase aims to transfer knowledge about processes and products from the development to the manufacturing phase.
This phase involves the following technical activities:
- Transfer of products from development to manufacturing
- For marketed products, transfer between manufacturing and testing sites
Commercial Manufacturing
The goal of the commercial manufacturing phase is to help pharmaceutical companies to manufacture products through a process of continual improvement and a state of control.
The pharmaceutical quality system should ensure high and uniform product quality, process performance, suitable controls, identification and evaluation of improvement opportunities, and knowledge growth.
The technical activities that companies must perform are:
- Acquire and control materials
- Provide facilities, equipment, and utilities
- Fabricate packaging materials and labels
- Store, release, or distribute the manufactured products
- Ensure quality control and assurance
Product Discontinuation
Product discontinuation activities aim to manage the final stage of the product lifecycle effectively.
During this phase, the following technical activities should be carried out:
- Management of document retention and sample retention
- Performing continual product evaluation and reporting
Managing the product lifecycle stage goals generates a high volume of documentation that must be handled appropriately to ensure compliance with ICH Q10.
SimplerQMS offers QMS software with robust document management capabilities that simplify the process of document creation, review, approval, retirement, and storage, promoting efficient collaboration among team members.
Our search and retrieval capabilities make it easy to locate and access relevant documents when needed. This ensures quick access to up-to-date information and enables timely responses to inquiries.
Management Responsibilities
Management responsibility states that leadership is essential in establishing and maintaining a company-wide commitment to quality, as per ICH Q10 section 2.
Effective leadership ensures that all company employees understand the significance of quality and are dedicated to upholding it throughout all aspects of the pharmaceutical quality system.
The management should provide guidance, allocate necessary resources, and create a culture that values and prioritizes quality.
Commitment
Management commitment is crucial for ensuring an effective pharmaceutical quality system. Senior management is responsible for establishing the system, defining roles and responsibilities, and ensuring their implementation throughout the company.
In addition, management should actively participate in several tasks, such as:
- System design and maintenance
- Provide support for PQS implementation
- Establish effective communication channels
- Define roles and responsibilities
- Conduct management reviews
- Promote continual improvement
- Allocate necessary resources
- And more
Quality Policy and Planning
Senior management should create a quality policy that outlines the company’s intentions and approach to quality.
The policy is an essential part of the QMS documentation and should encompass compliance with regulatory requirements, emphasizing the continual improvement of the pharmaceutical quality system.
Management should also define and communicate the quality objectives for implementing the quality policy.
Additionally, regular reviews and monitoring of key performance indicators (KPIs) of quality should be conducted.
Resource Management
Management should allocate adequate and appropriate resources to specific products, processes, or sites to ensure the effective implementation and maintenance of the pharmaceutical quality system.
Examples of resources include, but are not limited to, the following:
- Human resources
- Finances
- Materials
- Facilities
- Equipment
Internal Communication
Management should establish and implement effective communication processes within the company to ensure the flow of relevant information between all levels and departments.
These processes should also facilitate the appropriate and timely escalation of product quality and other issues when necessary.
Change in Product Ownership
During product ownership transitions, such as acquisitions, management should acknowledge the complexity of the situation and prioritize the seamless transfer of relevant information and processes.
In addition, it is important to identify and clarify the responsibilities of all companies involved in the process.
Pharmaceutical Quality Management System Elements
The four elements of a pharmaceutical quality management system, as outlined in ICH Q10 section 3.2, are the following:
- Process performance and product quality monitoring system
- Corrective action and preventive action (CAPA) system
- Change management system
- Management review of process performance and product quality.
These four elements work together to establish a comprehensive pharmaceutical quality system that helps ensure the production of safe and effective products while promoting continual improvement.
The PQS elements may be required in part by regional GMP regulations. Still, the ICH Q10 quality management system model aims to enhance them to promote a lifecycle approach to product quality.
Process Performance and Product Quality Monitoring System
Pharmaceutical companies should plan and implement a monitoring system to ensure control over process performance and product quality.
The monitoring system should ensure consistent product quality and identify opportunities for improvement.
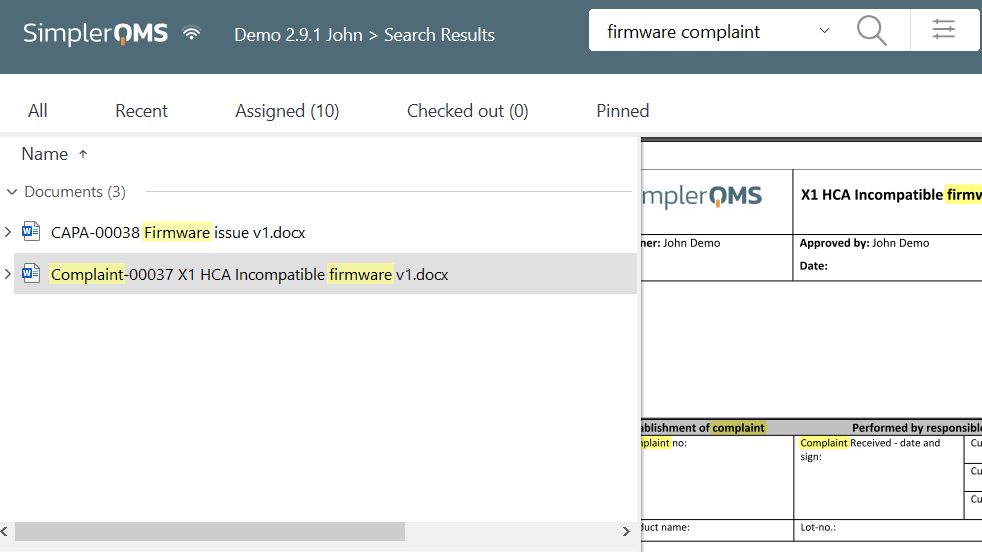
Handling process deviations to ensure performance and product quality effectively is essential.
Using an eQMS such as SimplerQMS with the Deviation Management module, companies can streamline the investigation, documentation, and resolution of issues.
Our software allows companies to relate deviation documents with products, manufacturing processes, and vendors, facilitating the tracking of related data and helping investigate root causes for the issue.
Corrective Action and Preventive Action (CAPA) System
Companies should have a system for implementing corrective and preventive actions (CAPA) based on various sources, such as complaints, deviations, recalls, audit findings, product monitoring data, and more.
Investigations should be conducted using a structured approach to determine the root cause. The level of investigation should be proportional to the level of risk, following ICH Q9 guidelines.
The CAPA methodology should lead to better understanding and improvements in products and processes.
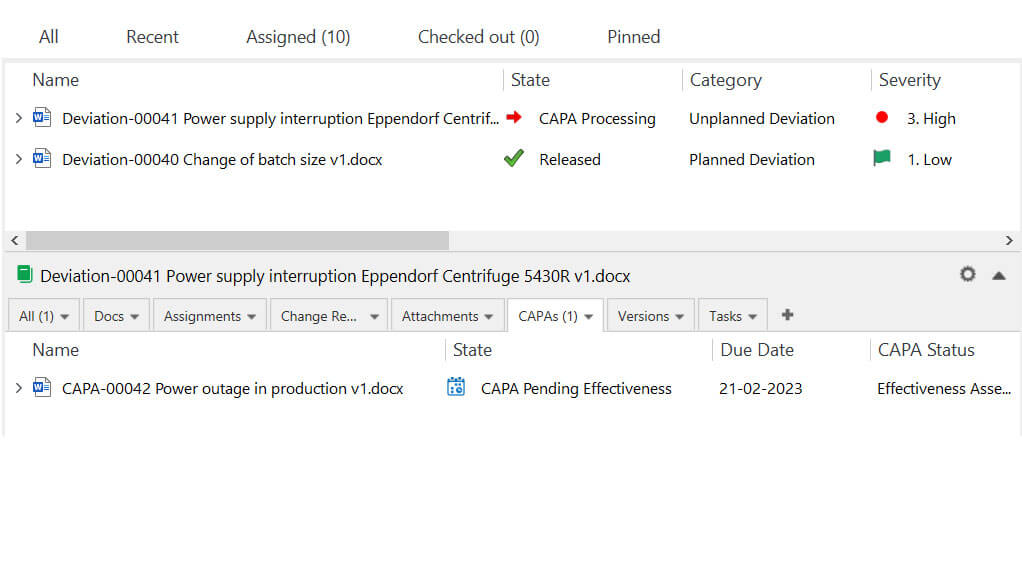
SimplerQMS solution also includes a CAPA Management module that simplifies and automates the corrective and preventive action process.
The software simplifies the escalation of recorded issues to CAPA processes allowing a seamless and closed-loop workflow.
Pharmaceutical companies can streamline CAPA activities for efficient and effective quality issue management, including data collection, routing, notifications, approvals, and follow-up.
Change Management System
An effective change management system should be in place to evaluate, approve, and implement changes properly.
Expert teams with relevant expertise and knowledge should evaluate proposed changes to ensure their technical justification.
After implementation, an evaluation should be conducted to confirm that the change met its objectives.
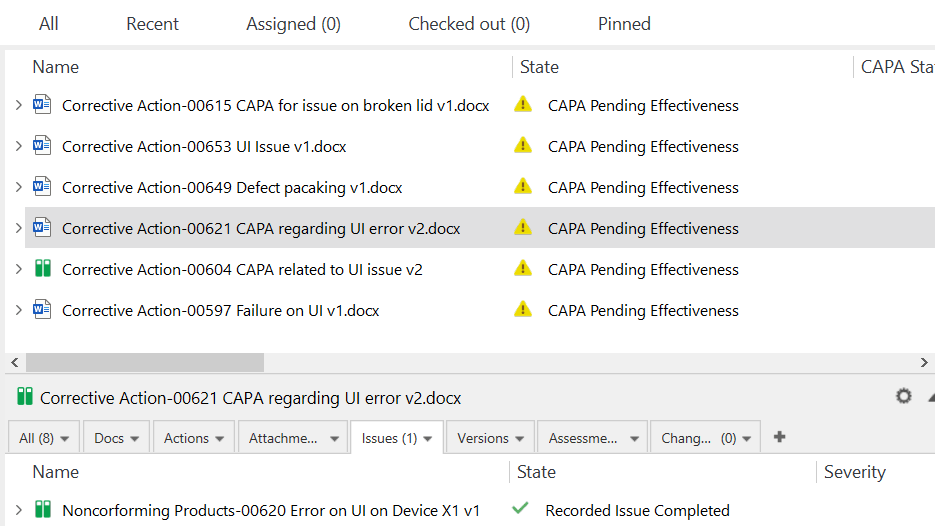
SimplerQMS offers robust Change Control Management capabilities enabling companies to create, document, and effectively manage all changes.
By utilizing our software, companies can benefit from automatic reminders and notifications that keep employees informed about change requests and their associated activities.
Management Review of Process Performance and Product Quality
Management reviews should ensure that process performance and product quality are effectively managed throughout the lifecycle.
The review process may vary in frequency and level of detail, depending on the company’s size and complexity. It should include efficient communication and escalation mechanisms to address quality issues and involve senior management for in-depth evaluation.
The management review should include the following:
- Review of results from regulatory inspections, audits, and commitments to regulatory authorities.
- Review of quality assessments of customer satisfaction, process, and product monitoring conclusions, and the effectiveness of changes.
- Review of follow-up actions implemented based on previous management reviews.
Knowledge Management and Quality Risk Management
Knowledge management and quality risk management are essential for effectively implementing the pharmaceutical quality system model, according to ICH Q10 section 1.6.
These practices serve as enablers, allowing companies to achieve the objectives outlined in ICH Q10 by facilitating science-based and risk-informed decisions regarding product quality.
What is Knowledge Management?
Knowledge management is the systematic acquisition, analysis, storage, and dissemination of information throughout a product’s lifecycle, from development to commercialization and even discontinuation.
It includes managing product and process knowledge gained through scientific approaches during development activities. Effective pharmaceutical document management processes are essential to ensure that all knowledge is recorded and readily available to relevant individuals.
Various sources contribute to this knowledge, such as:
- Prior knowledge
- Pharmaceutical development studies
- Technology transfer
- Process validation studies
- Manufacturing experience
- Innovation
- Continual improvement
- Change management activities
What is Quality Risk Management?
Quality risk management involves a proactive approach to identify, scientifically evaluate, and control potential risks to ensure product quality.
With SimplerQMS’s Risk Management module, companies can develop risk mitigation plans by defining control measures, implementing preventive actions, and establishing contingency plans.
The software provides a platform for documenting risk mitigation strategies and integrating them with other quality processes to facilitate traceability.
Continual Improvement of the Pharmaceutical Quality System
Continual improvement in the pharmaceutical quality system involves various activities to manage and enhance its effectiveness, as per ICH Q10 section 4.
These activities include ongoing evaluation, analysis, and implementation of improvements to ensure the system delivers uniform and high-quality pharmaceutical products.
Management Review of the Pharmaceutical Quality System
Management should conduct regular reviews of the pharmaceutical quality system using a formal process. These reviews should include evaluating the achievement of quality system objectives and assessing performance indicators that monitor the effectiveness of processes.
The PQS performance indicators cover areas such as:
- Complaints
- Deviations
- CAPA
- Change management
- Feedback on outsourced activities
- Risk assessments
- Internal audits
- Regulatory inspections
- Customer audits
- And more
The outcomes of management review and monitoring of the quality system may lead to several outcomes, including:
- Improvements to the quality system and processes
- Allocation or reallocation of resources
- Training of personnel
- Revisions to quality policy and objectives
- Documentation and communication of review results and actions
Additionally, any relevant issues identified during the review may be escalated to senior management for appropriate attention and resolution.
Internal and External Factors Impacting the PQS
Management should actively monitor various internal and external factors to ensure the effectiveness and adaptability of the pharmaceutical quality system.
Factors monitored by management can include:
- Emerging Regulations: Management should keep up with the latest regulatory requirements, industry guidance, and any emerging quality issues that could impact the PQS.
- Innovations for Enhancing the PQS: Management must be attentive to developments in technology, processes, and methodologies that can contribute to improving the systems.
- Changes in Business Environment: Management should monitor shifts in market trends, customer expectations, and internal organizational goals.
- Changes in Product Ownership: Management must monitor and assess any transitions in ownership to ensure the seamless integration of quality processes, responsibilities, and information.
Role of Pharmaceutical eQMS Software
Pharmaceutical electronic Quality Management System (eQMS) software plays an important role in supporting the implementation of the ICH Q10 guidelines.
The eQMS software can help pharmaceutical companies to improve the quality of their products and services and to comply with regulatory and customer requirements. It automates and streamlines quality processes, enhances communication and collaboration, and promotes a culture of continual improvement.
Although the ICH Q10 Quality System Model can be implemented using a manual paper-based or hybrid QMS, eQMS software provides further improvements.
Paper-based and hybrid systems, while suitable for small companies with sufficient resources, have inherent challenges and limitations. They are prone to errors and damaged documents and require physical space for storage, leading to inefficiencies and potential data loss.
On the other hand, eQMS software offers significant advantages in terms of efficiency, accuracy, and compliance. It provides a digital platform to create, store, and access documents electronically.
SimplerQMS provides fully validated pharmaceutical QMS software.
SimplerQMS offers all Life Science QMS modules integrated to optimize quality management processes. These include document management, CAPA management, change control, employee training, deviation, complaint, supplier, audit, risk management, and more.
Our system helps companies to achieve regulatory compliance with several Life Science requirements, such as GMP guidelines, ICH Q10, FDA 21 CFR Part 11, 210, and 211, EU GMP Annex 11, ISO 9001:2015, and many others.
To better understand the benefits of implementing an eQMS solution, we suggest downloading our eQMS Business Case template.
This valuable tool provides a structured approach for assessing the value of an eQMS tailored to your company’s needs. It also facilitates effective communication of your findings to management. Using a business case analysis, you can explore potential efficiency increases, cost savings, and compliance improvements.
Frequently Asked Questions
What is Q10 in ICH?
The Q10, in the context of ICH, refers to the quality guideline for the pharmaceutical quality system model. The ICH Q10 outlines requirements for implementing a pharmaceutical quality system to ensure the consistent quality of pharmaceutical products throughout their lifecycle.
What Is the Latest Version of ICH Q10?
The latest version of ICH Q10 is the Step 5 version, published in June 2008. Until this article’s publication date (July 2023), there have been no subsequent revisions to the guideline.
What Is the Scope of ICH Q10?
The scope of ICH Q10 is to guide for implementation of a quality management system in the pharmaceutical industry. It applies to the systems involved in developing and manufacturing pharmaceutical drug substances and drug products throughout their lifecycle.
Applying the elements outlined in ICH Q10 should be tailored to each stage of the product lifecycle, considering each stage’s unique characteristics and objectives. The product lifecycle encompasses pharmaceutical development, technology transfer, commercial manufacturing, and product discontinuation for new and existing products.
What Are the Objectives of ICH Q10?
The objectives of ICH Q10 aim to achieve three main goals.
Firstly, it aims to establish, implement, and maintain a system that delivers products meeting the quality requirements of customers and regulatory authorities. This objective emphasizes the importance of product realization.
Secondly, the model emphasizes developing and utilizing effective monitoring and control systems to ensure process performance and product quality. This objective aims to maintain a state of control throughout the processes.
Thirdly, the model promotes continual improvement. It encourages identifying and implementing improvements for consistent product quality, process efficiency, and innovation. Quality risk management is essential in prioritizing improvement areas within the system.
What Are the Benefits of Implementing the ICH Q10 Pharmaceutical Quality System?
The implementation of ICH Q10 can provide significant benefits to pharmaceutical companies. For instance, one of the most important benefits of ICH Q10 is to ensure uniform and high product quality and safety.
An effective Pharmaceutical Quality System (PQS) ensures the production of uniform and high-quality products that comply with regulatory requirements. This helps to prevent recalls, rework, and other quality issues, saving companies money and protecting patients.
Another benefit of ICH Q10 is cost reduction. ICH Q10 implementation helps to identify and reduce unnecessary costs associated with quality issues. With ICH Q10, companies improve efficiency and productivity, leading to further cost savings.
ICH Q10 helps to foster a culture of continual improvement by implementing risk management principles and tools to proactively identify, assess, and control risks across the product lifecycle.
Finally, ICH Q10 helps to improve regulatory harmonization. Compliance with ICH Q10 guidelines aligns companies with international guidelines, facilitating inspections and reducing the risk of non-compliance.
How Can Companies Ensure Compliance With the ICH Q10 Pharmaceutical Quality System?
Companies can ensure compliance with the ICH Q10 Pharmaceutical Quality System by following the requirements outlined in the guidance and implementing an internal audit process to ensure adherence to these requirements.
Here are some examples of key steps to ensure compliance with ICH Q10:
- Familiarize themselves with the ICH Q10 guidelines: Companies should thoroughly understand the requirements and recommendations outlined in the ICH Q10 guidelines.
- Conduct a gap analysis: Thoroughly evaluate the company’s existing quality management system against the requirements of ICH Q10. This gap analysis will help identify areas where the company’s current practices align with or deviate from the guideline.
- Implementing a risk-based approach to quality: Identify and assess risks to quality at all product lifecycle stages. Take steps to mitigate those risks.
- Ensure adequate training and awareness: Provide appropriate training to employees to ensure they understand the requirements applicable to their roles and responsibilities.
- Having a well-documented PQS: Have clear documentation describing the PQS and its implementation.
- Conduct regular audits and reviews of the PQS: Periodic inspections should be conducted to ensure that the PQS is effective and implemented correctly.
- Continually improve the quality system: Foster a culture of continual improvement by implementing corrective and preventive actions, analyzing quality data, conducting risk assessments, and actively seeking opportunities for improving the PQS.
What is the Difference Between PQS and QMS?
PQS (Pharmaceutical Quality System) and QMS (Quality Management System) are both quality management systems. The main difference between them is that PQS is specifically designed for the pharmaceutical industry, while QMS is a broader system applicable to any industry.
PQS refers to the quality system implemented by a pharmaceutical company to ensure the quality of its products throughout its lifecycle. It encompasses various elements, including process performance, product quality monitoring system, CAPA system, change management system, and management review, all aimed at achieving and maintaining product quality.
QMS is an overall quality system that defines the policies, processes, procedures, and responsibilities required to ensure that a company’s products or services consistently meet customer and regulatory requirements.
Final Thoughts
The ICH Q10 guideline, also known as the ICH Q10 Pharmaceutical Quality System (PQS), provides a comprehensive framework for pharmaceutical companies to effectively manage quality throughout the product lifecycle.
To implement an effective PQS model, pharmaceutical companies are increasingly adopting eQMS solutions. These solutions streamline quality processes and simplify compliance with regulatory requirements.
SimplerQMS offers an eQMS tailored specifically for pharmaceutical companies. The software automates and streamlines quality processes and supports compliance with ICH Q10 and other regulations, which include GMP, ISO 9001:2015, FDA 21 CFR Part 11, 210, 211, and more.
Want to learn more about how SimplerQMS can support your company QMS? Then book a personalized demo with our experts today and see how our eQMS solution can help you streamline and automate your quality management processes.