Let’s imagine that your medical device company, headquartered in Nurnberg, Germany, has designed an innovative and award-winning, extended vision foldable, presbyopia-mitigating, intraocular lens (IOL) for use by patients undergoing cataract surgery. This device will be your stepping stone into the lucrative United States of America market, which you haven’t yet targeted.
Upon doing some research online, you will come across the terminology “21 CFR Part 820 Quality System Regulation (QSR)”. Which is a set of regulations put in place by the US FDA that all medical device manufacturers must adhere to when they wish to sell their products in that country.
In this article, we will take a deep look at what 21 CFR Part 820 QSR is all about, its purpose, and requirements, and understand the role played by QMS software in supporting compliance with this important regulation.
As a QMS software provider, we at SimplerQMS help medical device companies of all sizes transition from traditional paper-based and hybrid QMS systems into purpose-built electronic QMS (eQMS) made specifically for medical device companies.
If you are interested in learning more about how our eQMS solution can help your organization streamline quality management processes and simplify compliance with 21 CFR Part 820, feel free to request a demo and talk to one of our experts today.
Jump to:
- What is FDA 21 CFR Part 820?
- Purpose of FDA 21 CFR Part 820 Quality System Regulation (QSR)
- FDA 21 CFR Part 820 Quality System Regulation (QSR) Requirements
- Simplify 21 CFR Part 820 Compliance with a Medical Device QMS Software
What is FDA 21 CFR Part 820?
The FDA 21 CFR Part 820 is a set of regulations from the United States Food and Drug Administration (FDA) that emphasizes current good manufacturing practice (cGMP) requirements.
All medical device manufacturers must follow 21 CFR Part 820 when they manufacture and sell medical devices for the US market. With this, the company assures both the public and the regulatory agency that the medical devices are safe and efficacious.
Do note that 21 CFR Part 820 covers the entire gamut of a product’s lifecycle – from its design, manufacture, packaging, and labeling, to storage, installation, and servicing of all finished medical devices that are to be used by humans. This also includes the facilities and designs that will be used for these processes.
What Is the Difference Between ISO 13485:2016 and 21 CFR Part 820?
The main difference between ISO 13485:2016 and 21 CFR Part 820 is that while ISO 13485:2016 is a voluntary international QMS standard for medical device companies, 21 CFR Part 820 is a compulsory QMS regulation that is specific only to medical devices sold in the United States.
General differences between these two standards are given in the table below.
| ISO 13485:2016 | 21 CFR Part 820 |
|---|---|
| Voluntary by nature. When a medical device company meets the requirements of ISO 13485, it is conforming to the standard. | Mandatory. When a medical device company meets the requirements of 21 CFR Part 820, it is complying with the regulation. |
| Risk management is emphasized. | Risk management is rarely emphasized. |
| Accepted across the globe. | Applicable only to the USA. |
| Multiple revisions since inception. | Structure unchanged since 1996. |
| Collaboratively created. | Created solely by US FDA. |
FDA 21 CFR Part 820 Amendment
The FDA is planning an amendment to 21 CFR Part 820, to align it more closely with ISO 13485:2016. This proposed regulation was published on 23 February 2022 and can be accessed here.
The Quality System Regulations (QSR) under 21 CFR Part 820, first brought out in 1978, were previously amended way back in 1996.
When finalized, the amendment will be a crucial step in the global harmonization of all medical device regulations. This harmonization will guarantee the removal of multiple regulatory requirements and impediments that are barriers to patient access to effective medical devices.
The new regulation will be titled Quality Management System Regulation (QMSR).
It will contain certain key elements of 21 CFR Part 820, including certain definitions, clarifying concepts, and additional requirements that will make sure that the new regulation continues to conform to the Federal Food, Drug, and Cosmetic Act (FD&C Act).
The latest version of ISO 13485 (namely ISO 13485:2016) will become a part of the proposed QMSR. Any future revisions to this ISO standard will only be incorporated if and when the FDA deems it necessary.
Recommended Reading: Medical Device Quality Management System (QMS)
Purpose of FDA 21 CFR Part 820 Quality System Regulation (QSR)
The purpose of the 21 CFR Part 820 (QSR) is to make sure that medical devices that have been created and developed for the US market are safe and efficacious. Medical device manufacturers must assure the regulatory agency (in this case the FDA) and the end-users that the products they manufacture are both effective, and safe for the intended purpose.
This regulation is mandatory, and any discrepancy or non-compliance can result in the FDA issuing Warning Letters, which will be shared with the public via the FDA website.
For instance, on November 4, 2022, the FDA issued a safety communication to warn parents, caregivers, and healthcare providers about unapproved infant head-shaping pillows that can result in an unsafe sleep environment.
FDA 21 CFR Part 820 Quality System Regulation (QSR) Requirements
As discussed earlier, the QMS requirements for any medical device company marketing in the US are given in 21 CFR Part 820 Quality System Regulation (QSR).
We will now look closely at the essential aspects that must be addressed by your company’s Quality Management System. Additionally, several examples are given to demonstrate how a modern electronic QMS (eQMS) can help you streamline these processes.
NOTE
Do note that the following details are, by no means, a comprehensive guide to FDA 21 CFR Part 820 QSR. Your medical device company must follow the official requirements laid out in the official document.
Subpart A – General Provisions
This section highlights the companies to whom this regulation applies, and relevant definitions, in accordance with the Federal Food, Drug, and Cosmetic Act.
This section clearly mentions that 21 CFR Part 820 is pertinent to all medical devices, either manufactured in the United States or imported and intended for use there.
Subpart B – Quality System Requirements
This section covers:
- Management responsibility
- Internal quality audits
- Personnel
Let’s look at each section in more detail.
Management Responsibility
This consists of rules and regulations about your company’s quality policies, resources, and planning.
- Quality Policy. Senior management of the company will commit to quality in accordance with 21 CFR Part 820. Ensure that all personnel within the company understand the requirements for such quality, and implement and sustain this quality.
- Organization. Senior management will establish and maintain a sufficient organizational structure. This means that they will provide appropriate responsibility and authority to key personnel for managing, performing, and assessing anything related to quality. Provide adequate resources including training. Appoint a key person who will supervise the quality system and provide feedback to the senior management at regular intervals.
- Management Review. At predefined intervals, management will review the effectiveness of the quality system. The entire process will be documented.
- Quality Planning. Your company should have a quality plan in place that will clearly elucidate the quality practices, activities, and resources that are relevant to the products that you design and manufacture.
- Quality System Procedures. The company will create protocols and procedures for the quality system.
It is crucial that you operate with a single source of truth that will ensure that decisions can be taken from data obtained from across the entire company. This is essential for compliance with regulatory commitments. One way of ensuring this is to have a centralized quality system that will act as the backbone of your quality management processes.
An eQMS software like SimplerQMS will enable you to have complete control over your quality data and documents, as well as simplify and automate most, if not all, of your quality processes. This will free up time for personnel to focus on other areas of the business while still maintaining compliance with 21 CFR Part 820.
Internal Quality Audits
Such audits are to be conducted by trained personnel who are not involved with a particular department/process. You will provide and conduct such quality audits to assure yourself that the quality system in the company is compliant with 21 CFR Part 820 QSR.
To be ready for audits, internal as well as external, you need to be able to have your documentation in order and ready to be retrieved at a moment’s notice to show the current state of compliance of your quality system.
This is where an eQMS with a built-in audit management module can help you.
By having all relevant documents in a central repository, you can save time and effort in retrieval, as well as have peace of mind knowing that you will not be caught off-guard during an audit.
Personnel
Your company must have experienced personnel to ensure that all quality-related activities are properly performed. When required, personnel are to be trained as per the requirements and such training must be documented.
When your medical device company has a high number of employees, and there are several branches located in different cities or countries, it becomes extremely difficult to manage and monitor the training of personnel.
An eQMS with a built-in training management module can automate and keep track of employee training, as well as maintain records of who has been trained on what (PDF documents, PowerPoint slides, videos, etc.), and when the training took place.
Furthermore, training-related KPI reports can be generated to provide visibility into the progress of employee training.
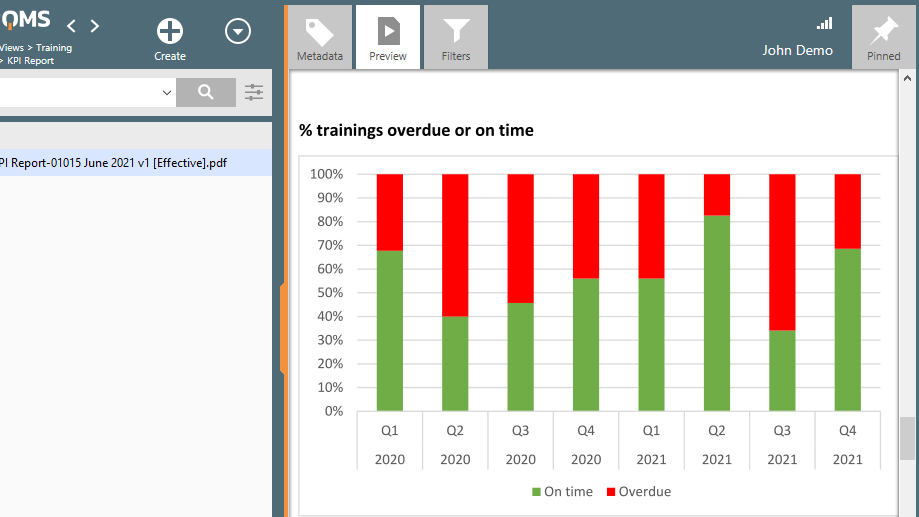
Subpart C – Design Controls
What is meant by design controls? To put it in simple terms, your medical device company must ensure that every step in the lifecycle of your products is following pre-defined processes and protocols.
Design controls apply to all Class II and Class III medical devices, and certain Class I devices.
The list of specific Class I medical devices includes:
- Tracheobronchial suction catheters
- Surgeon’s gloves
- Protective restraints, and so on
- As well as devices that are automated with computer software
As per this section of 21 CFR Part 820, you must have in place the following requirements:
- Design and Development Planning. You should have design and development plans for every medical device that you manufacture. These plans must be updated, reviewed, and approved when required.
- Design Input. Your company will ensure that the design of a particular medical device matches its intended use. Any incomplete, ambiguous, or conflicting requirements must be addressed. As with every other quality-related task, the design input requirements are documented, reviewed, and validated by authorized individuals.
- Design Output. You will establish and maintain suitable protocols to ensure that the design output will conform to the design input of every medical device that your company manufactures.
- Design Review. At suitable stages of a device’s design development, your company must have procedures for formal and documented reviews. The participant in these reviews will not only include concerned staff, but also staff without direct responsibility for that particular design stage, and specialists. The results will be documented in the Design History File (DHF).
- Design Verification. With design verification, you will have protocols that ensure that the design output conforms to the requirements of the design input.
- Design Validation: Your company will ensure that the devices, and their software, are compatible with their intended use. This will also include testing the production units via simulated or actual use conditions.
- Design Transfer. During this stage, you will ensure that the device design is properly translated into product specifications.
- Design Changes. You may wish to bring in some changes to the design of a medical device so that its efficiency and efficacy are improved. This is possible after review and approval at this stage.
- Design History File. The DHF for each type of medical device will show that the device was designed in agreement with approved design plans.
An eQMS software such as SimplerQMS can also help you manage design changes, as you will be able to track who made the change, when it was made, and why it was made. This will help you to prevent unauthorized changes from being made, and ensure that all changes are properly documented, reviewed, and approved.
Furthermore, with SimplerQMS you can easily compile your DHF, DHR, or DMR by using the Document Collection Tool. Just search, filter, and make a “snapshot” of the current documentation for each product, which you can then export or share externally.
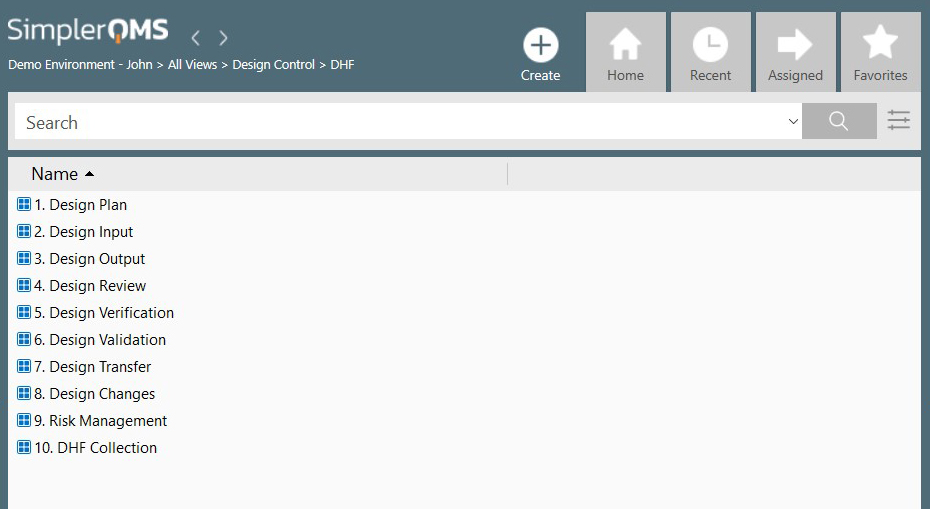
Recommended Reading: Design Controls for Medical Devices
Subpart D – Document Controls
A crucial aspect of your medical device company’s quality management system is document management.
This will include protocols for complying with document control as per 21 CFR Part 820 Subpart D, designating one or more personnel to review and approve documents and review changes as required. And, ensure that approved changes are communicated with required personnel in a timely fashion.
Furthermore, documents need to be named, numbered, and versioned, only the latest version must be circulated, and all older versions need to be archived.
SimplerQMS’s document controls offer an automated and streamlined way to manage documents and changes. With every change that is made, a new version is created automatically and all affected documents are updated accordingly. This ensures that everyone is always using the latest version of a document and that older versions are archived and easily accessible.
Recommended Reading
- Medical Device Document Control: What It Is & How to Simplify It
- Quality Management System Documentation [Best Practices]
Subpart E – Purchasing Controls
Purchasing controls are the management of your company’s supply chain and all third-party suppliers, contractors, and consultants.
You need to have in place all requirements, including quality requirements, which these third-party suppliers must meet before onboarding. You also need to define the type of control that you will have over the products or services provided.
And of course, you will need to document all records and documents and manage purchasing data.
Your company will need to define and document the procedures for qualifying suppliers, based on their ability to meet your quality requirements. As well as establish criteria for re-evaluating supplier performance.
SimplerQMS’s supplier quality management module is designed to help streamline and simplify your company’s supplier management processes. You can use it to manage your Approved Supplier Lists (ASLs), and other supplier-related documentation, monitor supplier performance, and track and approve changes.
This module integrates with the non-conformance and CAPA modules, so you can quickly and easily identify and address any issues that arise.
Recommended Reading: Medical Device Supplier Management Process (8 Steps)
Subpart F – Identification and Traceability
With identification procedures in place, you will ensure that there is no mix-up during the receipt, production, distribution, or installation of a medical device.
You should be able to identify every unit, lot, or batch of a finished device that is capable of causing considerable injury if it malfunctions.
Corrective actions must also be incorporated and documented in your Device History Record (DHR).
Document linking is a key function of QMS software solutions like SimplerQMS that ensures full traceability throughout your quality management system. With document linking, you can quickly and easily see the relationship between documents, and trace changes back to their source.
Subpart G – Production and Process Controls
With production and process controls, you will cover the inspection, measurement, test equipment, and process validation. You will also need to have protocols for corrective actions if required.
The company will also look into the following aspects:
- Environmental Controls. Any significant environmental conditions that can affect your product’s quality need to be controlled.
- Personnel. You will ensure that product quality is not compromised because of the health, cleanliness, personal practices, or clothing of your staff.
- Contamination Control. Ensure that all manufacturing equipment and products are sterile.
- Building. The buildings wherein the manufacturing processes take place must have sufficient space.
- Equipment. All manufacturing equipment must adhere to certain prerequisites. You will need to set schedules for maintenance and periodic inspections.
- Manufacturing Material. If there is reasonable doubt that a manufacturing material will adversely affect a product’s quality, such material must be discarded appropriately.
- Automated Processes. All computers and automated data processing systems that are part of the QMS or product production must be validated before use. Also, any changes in the software must have prior validation and approval. All validation activities and results need to be documented.
Subpart H – Acceptance Activities
Section H of 21 CFR Part 80 explains acceptance activities.
Your company’s QMS must provide details regarding:
- Protocols for inspection
- Tests
- Acceptance of incoming products
- In-process acceptance
- Acceptance of the finished product
Subpart I – Nonconforming Product
The company’s QMS must have procedures and protocols to identify whether products conform to acceptance criteria.
This conformance is not just with finished products but throughout the product’s lifecycle. It will include manufacturing, packaging, labeling, installation, and servicing.
For instance, you are not only responsible for the manufacture of the intraocular lens mentioned earlier, but also for its sterile packing, labeling, and distribution to your customers.
When a product does not conform to any aspect during its lifecycle, you will need to determine whether an investigation is necessary, and then proceed with the non-conformance.
When your company has a large portfolio of products and a big customer base, analyzing non-conformances becomes difficult.
QMS software with built-in non-conformance (NC) management capabilities can help automate and streamline the process of logging, analyzing, and investigating NCs by storing all relevant information in one central location. Pre-configured workflows and forms can also help ensure that investigations are conducted promptly and efficiently. KPI reports can provide valuable insights into areas where improvements are needed.
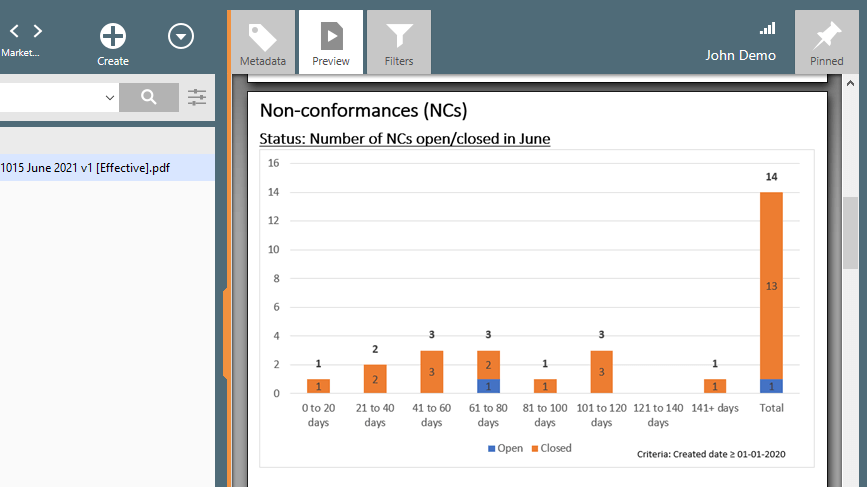
Recommended Reading
Subpart J – Corrective and Preventive Action (CAPA)
With Corrective Action and Preventive Action (CAPA), your medical device company will map out corrective and preventive actions for potential risks and errors.
Your QMS should cover all processes including audit findings, records, complaint handling, and returns.
For instance, if you identify a potential issue during an audit, you can take preventive action to ensure that the problem does not occur.
This could involve changing the design of your product, updating your quality control procedures, or implementing new safeguards in your manufacturing process.
CAPA management is a continuous process, and you will need to track all corrective and preventive actions to ensure that they are completed effectively.
CAPA management capabilities built into most eQMS solutions can help automate the CAPA process by providing pre-configured workflows and forms for logging, investigating, and taking corrective or preventive action. Plus, features like automated reminders, email notifications, escalation of open CAPAs, and KPI reports, can help ensure that CAPAs are addressed promptly and effectively.
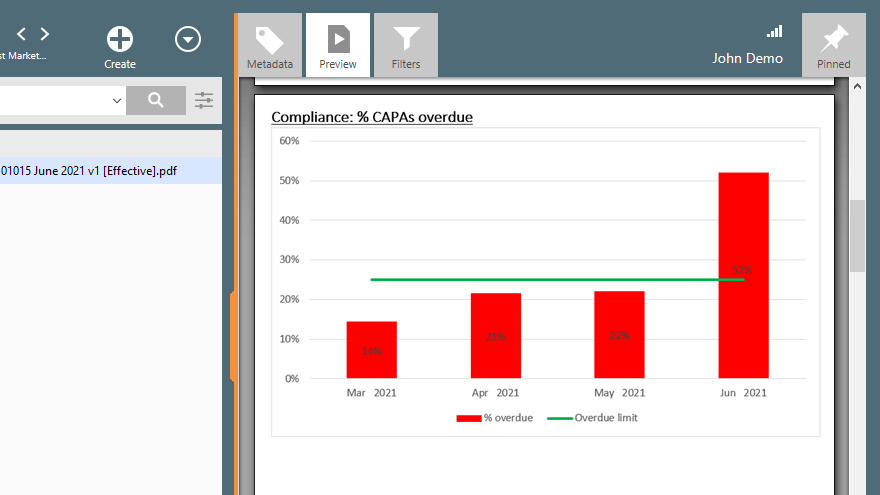
Recommended Reading: What Is CAPA in the Medical Device Industry?
Subpart K – Labeling and Packaging Control
With this set of controls, you will ensure that the products that the company manufactures are labeled clearly and affixed properly.
You will not want customers to raise complaints about illegible labels, or for that matter, complain that they have received products without any labels! It would be equal if products are wrongly labeled.
Do have a proper set of procedures for the production and inspection of labels and packages!
Subpart L – Handling, Storage, Distribution, and Installation
Another important aspect that 21 CFR Part 820 emphasizes is that your company will ensure that mistakes do not occur when finished products are being handled, stored, distributed, or installed.
These are amongst the last steps in the long lifecycle of any medical device. You will definitely not want any problems to arise at this point in time.
This only goes to show the importance of quality throughout the lifecycle of every medical device.
Subpart M – Records
Your company will need to maintain and make available the following records during audits.
- Device Master Record (DMR). The DMR will contain pertinent information on each device and product specifications, including quality assurance procedures.
- Device History Record (DHR). The DHR will contain the dates on which a device was manufactured, the quantity manufactured, and the quantity released.
- Quality System Record. The QSR will detail the locations wherein procedures and activities are stored.
- Complaint Files. Whenever the company receives complaints regarding one of its products, the company will review, evaluate, and maintain them in complaint files.
Record maintenance is a critical aspect of quality management, and most QMS software solutions offer powerful features for managing such records.
Subpart N – Servicing
When a medical device that you manufacture needs servicing, you should provide detailed instructions for the same.
You need to keep records of the location of such servicing, the personnel carrying out the servicing, and details of what is done. The same holds good even if these activities are outsourced to a third party.
Subpart O – Statistical Techniques
Where required, your company will have procedures for identifying statistical techniques for process capability and product characteristics.
Considering that statistical techniques are crucial for the service reports in 21 CFR Part 820, you should have in place innovative statistical methodologies that will give you an advantage during clinical studies.
Simplify 21 CFR Part 820 Compliance with a Medical Device QMS Software
You will now have a fair idea of the requirements of 21 CFR Part 820 that your medical device company must comply with. You should also have realized by now that a medical device QMS software solution will be of great help in complying with these regulations.
A system that offers comprehensive features aligned with the requirements of 21 CFR Part 820 will help ensure compliance while also providing other benefits like shortening time to market, reducing costs, and improving customer satisfaction.
The best QMS software solutions on the market today offer a range of features that support compliance with 21 CFR Part 820.
SimplerQMS is one such solution. It is an all-in-one medical device quality management software that helps you with design control, document control, non-conformance, complaint, CAPA, training, supplier management, and much more.
Time-stamped audit trails, electronic signatures, pre-configured workflows, procedure, and form/template package are just some of the features that make compliance easier. Work with your documents using familiar Microsoft Office tools, and save your documents into the cloud-based system with a single click. No need to download and upload documents every time you need to work on them!
SimplerQMS provides a fully validated system according to GAMP5. It fully complies with ISO 13485:2016, 21 CFR Part 11 and 820, and the EU Annex 11 regarding validation and electronic signatures. Plus, we perform continuous re-validation so that you don’t have to.
If you are considering whether an investment in a quality management solution will be beneficial for your company, then we recommend you get our free eQMS Business Case template to calculate the economic impact of investing in a quality management solution, and present it to the management for approval.
Final Thoughts
When your medical device company wishes to manufacture and market in the United States, you must comply with the Food and Drug Administration’s 21 CFR Part 820, which emphasizes quality management.
Failure to comply with these requirements can result in serious penalties, including recalls, loss of market approval, and even criminal charges.
Traditional methods of compliance with 21 CFR Part 820 are paper-based and often involve maintaining physical files for different elements like design control, documents, training, change management, etc.
This can be extremely tedious and time-consuming. Moreover, it is easy to lose track of critical documents and records when they are in physical form.
A medical device QMS software solution can help you automate and streamline your quality management processes, and make it easier to comply with the 21 CFR Part 820 requirements.
SimplerQMS offers quality management software that offers a comprehensive suite of features to support compliance with 21 CFR Part 820. If you are interested in learning more about our software, schedule a demo with one of our quality solution specialists today!