21 CFR Part 11 Compliance Software for Life Sciences
Ensure compliance with FDA 21 CFR Part 11 with robust electronic records and signature capabilities. Streamline QMS processes while maintaining data integrity and traceability.
TRUSTED BY
























21 CFR Part 11 Compliant Software With Broad QMS Process Support
Ensure compliance with FDA 21 CFR Part 11 using a fully compliant QMS software by SimplerQMS. We support companies in achieving compliance with FDA 21 CFR Part 11 and many other Life Science requirements, such as ISO 13485:2016, ICH Q10, GxP, FDA 21 CFR Part 210, and more.
The software provides streamlined procedures and controls to maintain electronic records’ authenticity, integrity, and confidentiality. Utilize electronic signatures with the same level of trust and legitimacy as handwritten signatures.
SimplerQMS provides a comprehensive eQMS solution, which includes all Life Science QMS modules, such as document management, change control, CAPA, training, audit management, and more.
Control User Access With Secure User Access Controls
Limit system access to authorized individuals and prevent unauthorized access to sensitive information.
Protect your data privacy and confidentiality using proper security measures, such as multi-factor authentication and periodic password updates.
Manage license assignments and access to SimplerQMS through Microsoft Entra ID (previously known as Microsoft Azure Active Directory), a cloud-based identity and access management service.
Use Microsoft Office To Work on Documents
Create and edit documents in familiar Word, Excel, and PowerPoint applications. Collaborate on documents across teams and departments using a cloud-based system.
Use our pre-configured workflows to streamline document creation and editing processes, facilitating document routing, approval, and retiring.
Optimize document creation using SimplerQMS complementary form and document templates based on Life Science requirements.
Implement a Fully Validated Software System
Software validation is a requirement in FDA 21 CFR Part 11, according to 21 CFR Part 11.10(a). SimplerQMS provides a fully validated system – validated according to GAMP5.
Avoid spending time and resources on software validation. SimplerQMS solution is re-validated upon the creation of a new version or upon applying standard updates.
We do all the validation work for you, so you don’t have to.
Utilize Robust Document Control Capabilities
Access documents effortlessly from any device at your convenience. Store all documents in a single and secure location using a cloud-based system.
Facilitate efficient collaboration and seamless information sharing among team members, promoting better teamwork and productivity.
Gain a comprehensive overview of all documentation by utilizing customizable document views.
Streamline document workflows using a 21 CFR Part 11 compliant DMS to control and manage documents efficiently in compliance with requirements outlined in 21 CFR Part 11.10.
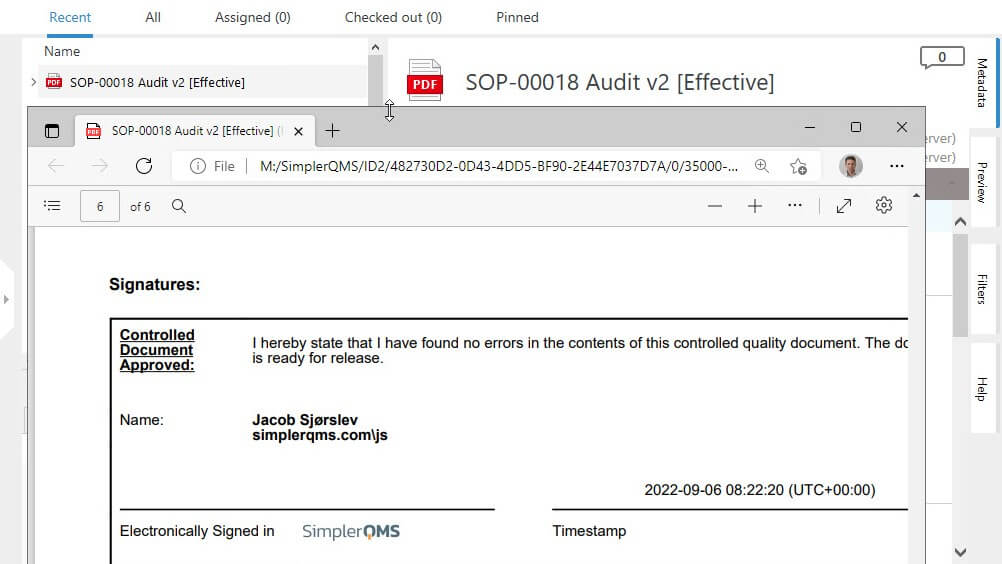
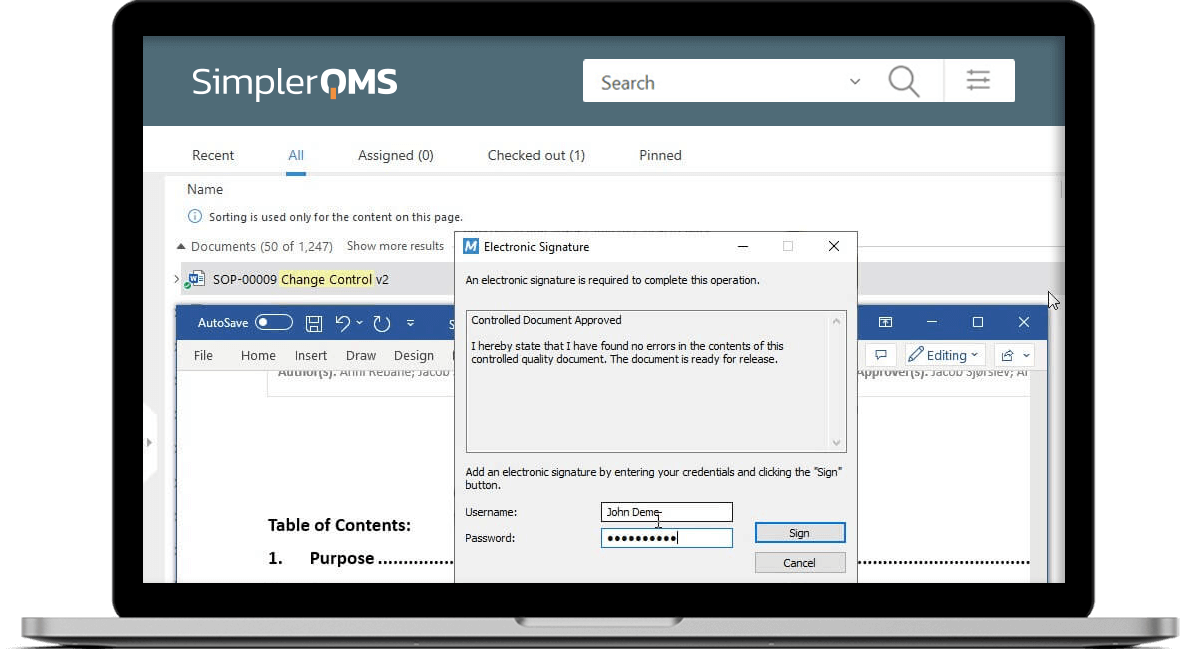
Ensure Document Integrity With eSignature
SimplerQMS provides 21 CFR Part 11 compliant electronic signatures.
Ensure document authenticity and integrity using electronic signatures with robust security measures, guaranteeing that your records remain tamper-proof and reliable. Enable a legally binding signing process that meets compliance with applicable electronic signature regulations.
Automatically capture essential details such as the signer’s name, date, time, and the meaning of the signature in a comprehensive audit trail.
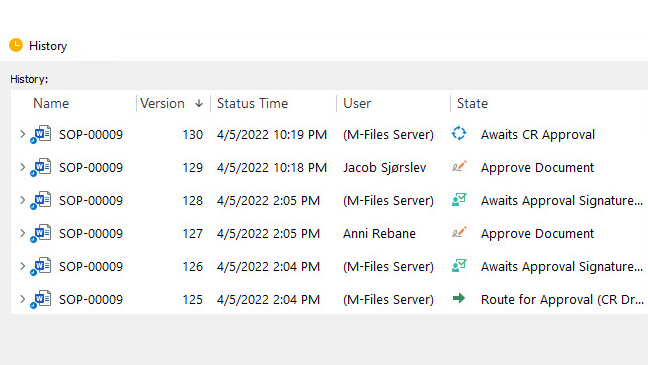
Monitor Document Activities With Detailed Audit Trails
SimplerQMS provides 21 CFR Part 11 compliant audit trails allowing you to monitor and record all document-related actions for accountability, transparency, and traceability.
Maintain a comprehensive time-stamped audit trail that automatically captures all the necessary information as outlined in 21 CFR Part 11 audit trail requirements.
Showcase a well-documented audit trail to external auditors, regulatory agencies, or internal stakeholders to show evidence of who did the change, to what, when, and why.
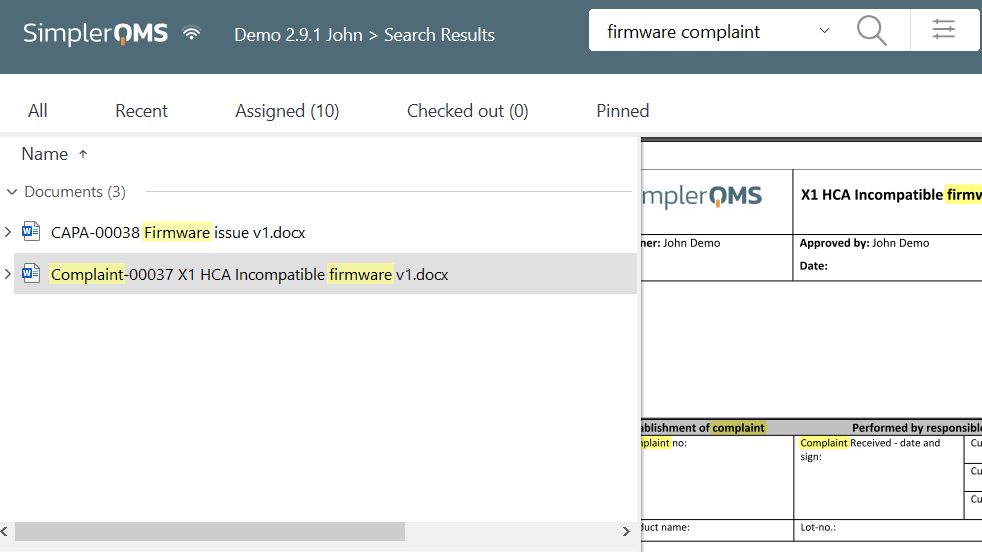
Archive and Retrieve Documents Efficiently
SimplerQMS automates the archiving of documents. Whenever a new version of a document is released, the previous one is automatically retired, archived, and watermarked for easy identification and retrieval as per requirements in 21 CFR 11.10(c).
Simplify document retrieval processes with a keyword search feature. Enable quick and efficient access to records through well-organized document repositories and easy access to critical information.
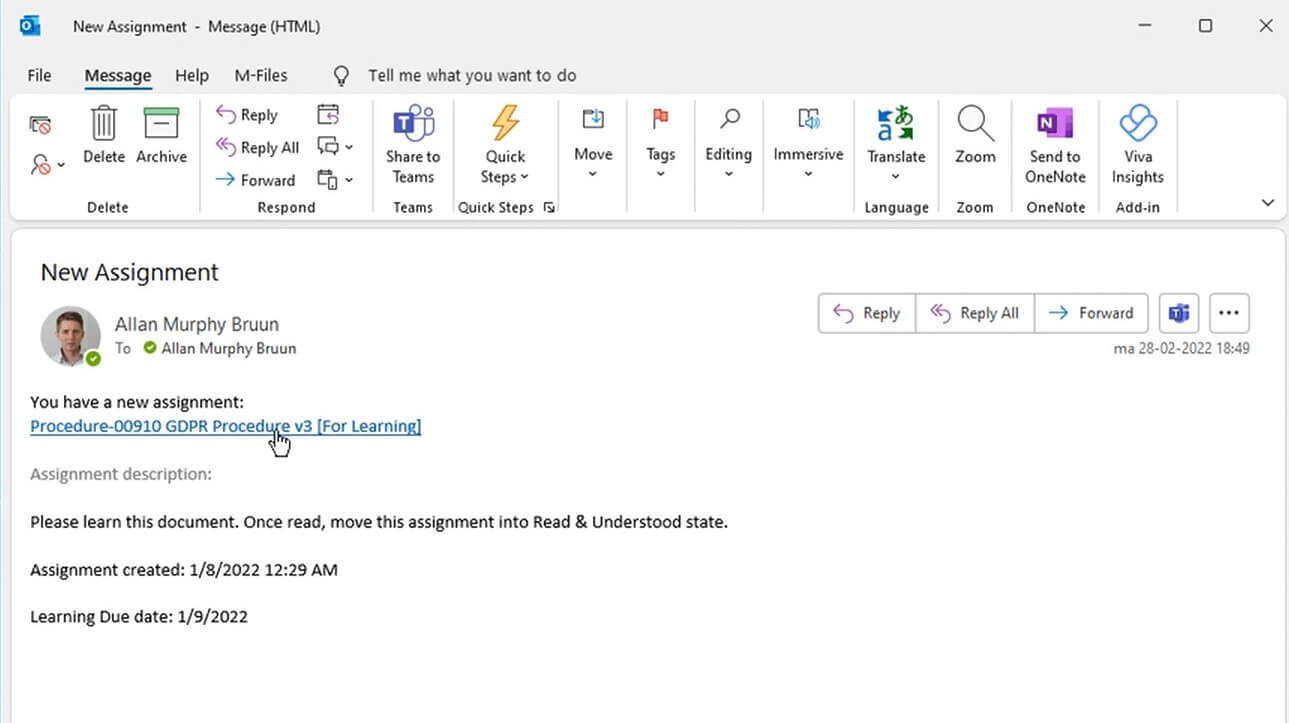
Get a Comprehensive System Training
A determination that persons using electronic record/electronic signature systems have sufficient training to perform their assigned tasks is a requirement outlined in 21 CFR 11.10(i).
With SimplerQMS you receive personalized training sessions from SimplerQMS Implementation Team to equip users with the necessary knowledge and skills to effectively utilize the system. Learn how to set up and manage workflows, ensuring the smooth and efficient execution of quality management processes.
Once implemented, benefit from our powerful training management module, which offers automatic notifications and reminders, ensuring timely and efficient training completion in your company.
Ensure Unique and Secure Passwords
SimplerQMS complies with requirements regarding controls for passwords and identification codes.
Employ unique passwords and identification codes for each user as outlined in 21 CFR 11.300. Ensure that access to the system is granted only to authorized individuals and prevent unauthorized access.
Periodically change passwords to prevent password aging and enhance security.
Enable multi-factor authentication, adding an extra layer of security. Require users to provide supplementary verification beyond their login credentials for a high level of security.
Streamline Workflows With Integration to Broader QMS
Seamlessly integrate with other QMS processes.
Keep all related documents connected and easily accessible with interlinked QMS modules. Escalate customer complaints to the CAPA process, link equipment to products, link suppliers to audits, and much more.
Integrate with other systems, such as CRM, ERP, LMS, MES, PLM, and other computer systems with SimplerQMS through open API and plugins, if necessary.
See Our SimplerQMS Solution in Action
Watch this brief video on how SimplerQMS simplifies document signing with 21 CFR Part 11 compliant electronic signatures.
Take a look at our automated workflows, notifications, and reminders.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Beyond Just 21 CFR Part 11 Compliance
Change Management
Ensure effective implementation of changes with a comprehensive change management process.
Training Management
Manage employee training by creating training plans, automating notifications, and tracking training progress.
Audit Management
Optimize audit management by efficiently scheduling and managing internal, external, supplier, and regulatory audits.
Supplier Management
Streamline supplier-related activities and manage suppliers in accordance with specific Life Science requirements.
Nonconformances
Track, manage, and address nonconformances efficiently, implementing effective solutions for improved quality and compliance.
CAPA Management
Address and solve issues by implementing preventive and corrective actions. Ensure effective resolution and long-term prevention.
Frequently Asked Questions
What is 21 CFR Part 11 Compliant Software?
A 21 CFR Part 11 compliant software complies with the requirements for electronic records and signatures outlined in the FDA 21 CFR Part 11.
The software provides procedures and controls to ensure the authenticity, integrity, and reliability of electronic records, as well as the security and validity of electronic signatures.
What 21 CFR Part 11 Compliant System Should Be Able to Do?
A 21 CFR Part 11 compliant system should be able to fulfill the requirements to ensure compliance with the regulation.
Here are some key aspects that a system should be able to do:
- Provide a validated system to ensure accuracy, reliability, and consistent intended performance.
- Prevent unauthorized access to the system.
- Ensure the security and integrity of electronic records.
- Enable the creation, modification, retrieval, and retention of electronic records.
- Support electronic signatures to ensure their trustworthiness and equivalence to handwritten signatures.
- Generate time-stamped audit trails to track and document system activities.
- Provide training to ensure users have the necessary knowledge to perform their tasks.
Who Needs to Use a 21 CFR Part 11 Compliant System?
A 21 CFR Part 11 compliant system is required for companies using electronic records and signatures that operate within FDA-regulated industries, such as medical device, biotechnological, CRO, and pharmaceutical.
Using the system is not necessary for companies that employ manual processes. However, using an electronic software solution dramatically increases efficiency and provides several other benefits.
What Are the Benefits of Using 21 CFR Part 11 Compliant System?
Using a 21 CFR Part 11 compliant system offers several benefits to companies operating within FDA-regulated industries.
Some of the key benefits include:
- Enhanced data integrity and security
- Regulatory compliance with FDA requirements
- Optimizes workflow efficiency
- Efficient employee training and collaboration
- Improves audit trail and traceability
- Streamlined and automated record-keeping processes
Does a 21 CFR Part 11 Software System Need to be Validated?
A 21 CFR Part 11 computer software system needs to undergo validation. Validation ensures a system operates according to its intended purpose and complies with regulatory requirements.
Software validation involves executing test scripts and documenting the entire process according to Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) procedures.
SimplerQMS provides a fully validated eQMS solution. We continually perform re-validation with each new version and standard update.
This means that we take care of all the software validation, eliminating the need for any additional costs, resources, or time commitments on your part.
To learn more about a QMS software system and when validation is necessary, refer to our article on QMS software validation.
How Much Does 21 CFR Part 11 Software Solution Cost?
The solution’s total cost depends on the number and type of licenses acquired.
The subscription covers all modules, hosting, system validation, implementation, user training, and ongoing support.
SimplerQMS provides a comprehensive eQMS solution that fully complies with 21 CFR Part 11. Our software includes all Life Science QMS modules, which cannot be acquired individually.
With our solution, you receive everything and have the flexibility to decide which modules to implement and when to do so.
For detailed information about SimplerQMS pricing and the features and services included, please visit our pricing page.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.