The 9 Best QMS Software Solutions for Life Sciences
Quality Management System (QMS) software is a digital tool designed to manage and streamline quality processes within a company. Choosing the best QMS software is an essential task that demands thorough research and careful consideration.
Our expert research team devoted several hours to analyzing the top-tier quality management software for Life Sciences. We provide comprehensive information on QMS software solutions and how to choose the most suitable QMS software for medical device, pharmaceutical, and other life science companies.
Discover SimplerQMS fully validated eQMS software designed for Life Sciences.
What Is the Best Quality Management Software for Life Sciences?
The 9 best quality management software for Life Sciences are listed below.
The list of the best QMS software vendors is based on extensive knowledge from our quality experts, our customers’ and partners’ feedback, comprehensive research, and an analysis of our experience compared to frequently referenced industry vendors.
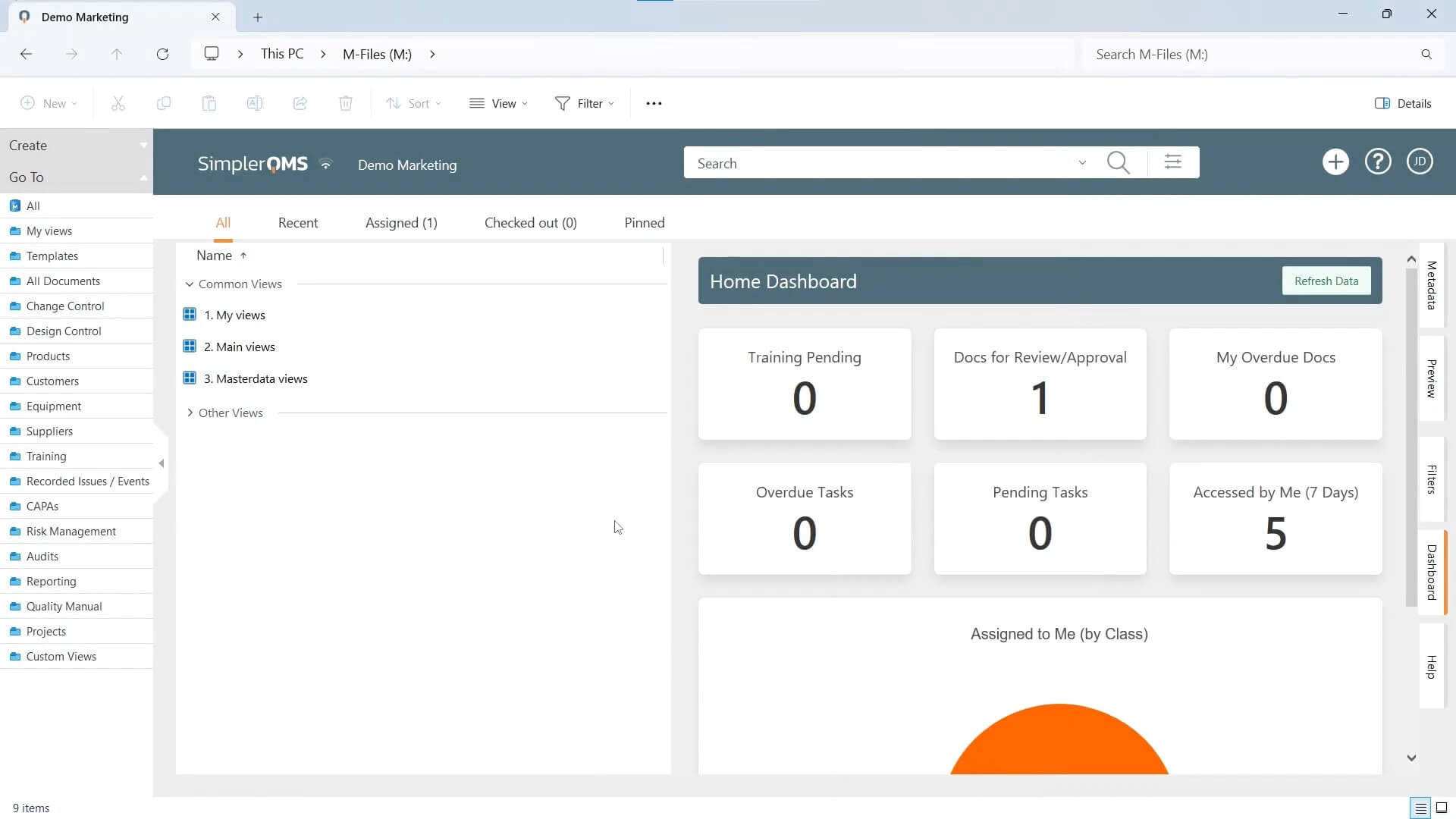
SimplerQMS
Deployment: Web, iOS, Android, Windows
SimplerQMS offers a cloud-based QMS software solution that is fully validated according to GAMP 5. SimplerQMS is designed for companies in the Life Science industries, such as medical devices, pharmaceuticals, laboratories, and biotechnology.
SimplerQMS provides comprehensive support to all QMS processes, such as document control, change control, training management, nonconformance and deviation management, CAPA management, design control, audit management, supplier management, equipment management and calibration management, and more. The system also provides quality KPI reporting capabilities.
By employing established workflows, users are guided through the quality processes with improved efficiency and compliance. The software provides automated notifications and reminders to ensure the timely completion of review, update, and approval tasks.
Native Microsoft suite integration enables seamless document editing and collaboration using familiar tools like Word, Excel, and PowerPoint. SimplerQMS also provides a template package that includes a quality manual, procedures, forms, and instructions. All these templates are based on Life Science requirements. If you do not already have a QMS in place, these templates can serve as an initial starting point. Additionally, the system allows for the use of existing document templates.
SimplerQMS software complies with the regulatory requirements related to the application of computer software used in the QMS in ISO 13485:2016, FDA 21 CFR Part 11, 211, 212, and 820, EudraLex Volume 4 GMP Part I, and EudraLex GMP Annex 11.
Furthermore, the system supports companies in achieving and maintaining compliance with several Life Science requirements, such as GxP, ISO 9001:2015, ISO 13485:2016, ISO 15189:2022, MDR, IVDR, 21 CFR Part 210, 211, 212, and 820, ICH Q10, and others.
SimplerQMS’ QMS software is fully validated according to ISPE GAMP 5 and re-validated upon the creation of a new version or upon applying standard updates.
We are responsible for all software validation procedures, like Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). There are no additional expenses, resources, or time commitments to software validation on the customers’ part.
SimplerQMS is available as an all-inclusive annual subscription, with pricing determined by the number of user licenses. The licenses encompass a range of user categories, including users for Read & Sign licenses, Single-User licenses, and Shared licenses.
The subscription includes implementation, unlimited training, validation, continuous re-validation, hosting, audit assistance, and access to all QMS modules, all at a single price.
Training is delivered through online meetings, and users can access an extensive knowledge base with guides to facilitate the learning process and serve as a reference for system usage.
The implementation process usually takes 5 to 6 weeks and is divided into three phases, each focusing on deploying specific modules.
SimplerQMS provides a no-cost trial environment where potential customers can explore the software’s capabilities and gain firsthand experience before making a purchase decision.
SimplerQMS offers a range of integration options, including APIs, ETL (Extract, Transform, Load) for import and export, plugins, and middleware connectors. These options facilitate data integration with various other software systems, such as Enterprise Resource Planning (ERP), Learning Management Systems (LMS), Customer Relationship Management (CRM), Computer-Aided Design (CAD), and Computer-Aided Manufacturing (CAM), among others.
Based on customer reviews on software review platforms like Capterra, GetApp, and Software Advice, SimplerQMS is best known for its ease of use and good quality-to-price ratio. Users appreciate the software’s user-friendly features, which make it easy to learn and use even for non-technical users.
A significant majority of customer feedback mentions the outstanding performance of the SimplerQMS customer support team. Users praise the team’s deep understanding of the system and responsiveness in addressing questions and concerns.
What Are the Key Considerations Working With SimplerQMS?
Key considerations for working with the SimplerQMS solution are presented below.
SimplerQMS is a fully validated system that offers configurability within a validated framework. This framework aligns with requirements from standards such as GxP, ISO 13485:2016, 21 CFR Part 11, EU Annex 11, and others. Its design enables rapid implementation and removes the need for additional validation from the customer’s perspective.
The software is a ready-to-use system designed for immediate deployment in life science organizations that need industry-specific process support. However, users requiring high levels of customization may find this solution less beneficial.
SimplerQMS integrates with Microsoft Office applications, allowing users to work with familiar tools. However, this solution might not be the best fit for users who are against utilizing Microsoft Office.
SimplerQMS offers personalized training sessions and unlimited support delivered by system experts via online meetings, ideal for companies seeking a tailored learning experience.
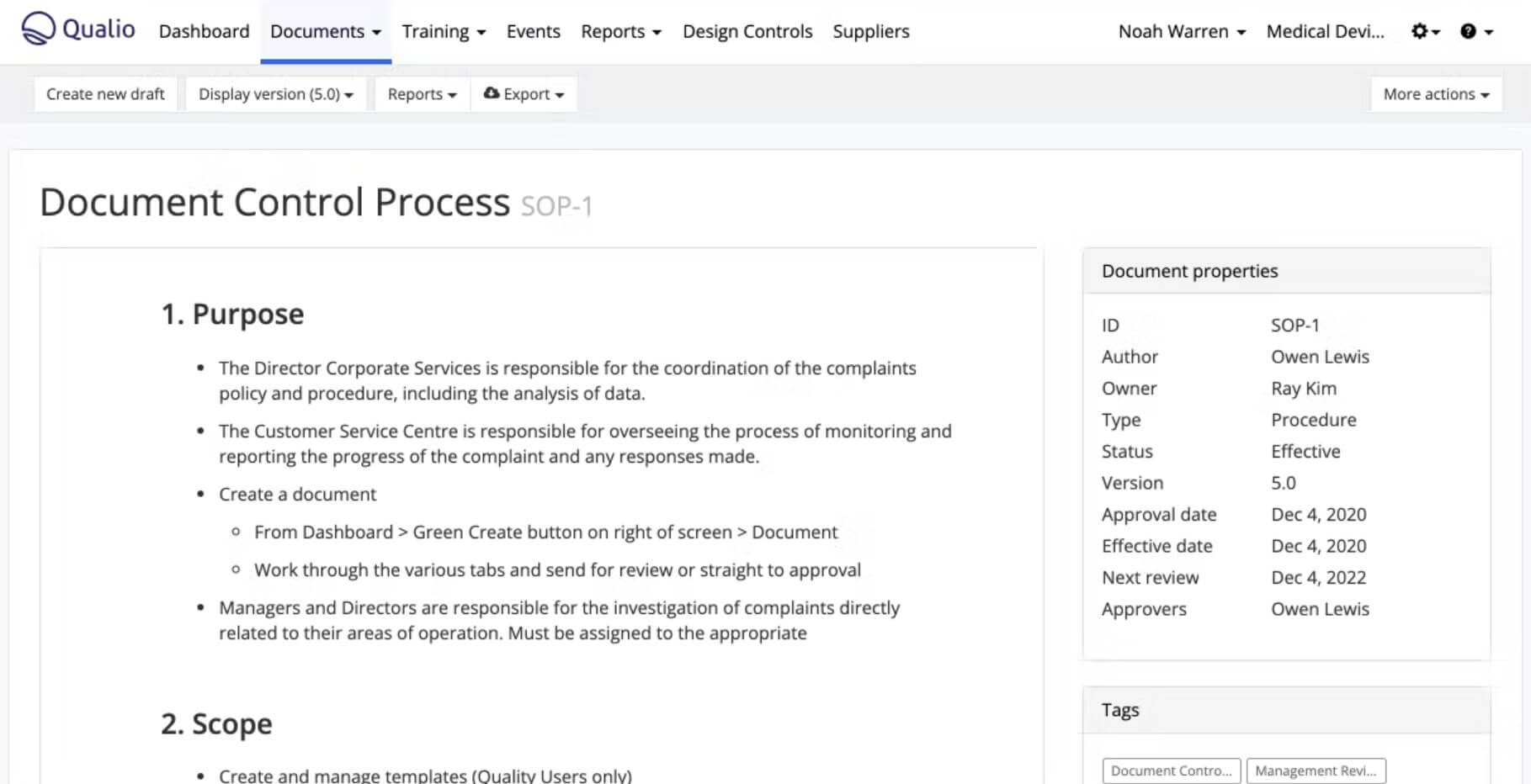
Qualio
Deployment: Web
Qualio is a holistic quality management platform for companies in the medical device, pharmaceutical, and biotechnology industries. Their target customer segment is growing Life Science companies and startups.
Qualio solution provides core QMS processes, including document management, design controls, risk management, training, change control, audit management, and more.
However, it is noteworthy that the system does not provide equipment and a calibration module.
With Qualio’s native web-based document editor, you can create, review, and comment on controlled documents and records, fostering a transparent document management process.
The software aligns with the latest regulatory standards, such as FDA 21 CFR Part 11, ISO 13485:2016, ICH Q10, GxP, FDA 21 CFR Part 820, and more. It is ISO 27001:2022 certified and validated according to ISPE GAMP 5.
Regarding validation, users still need to qualify Qualio as a supplier of software as a service (SaaS), plan and define the approach to computer software assurance activities, evaluate business processes and risk assessment, and prepare the Validation Summary Report (VSR).
The software implementation process is divided into four phases: orientation, validation, migration, and training. A dedicated customer onboarding manager guides you through each phase to ensure a seamless transition.
Qualio is provided to customers on an annual recurring subscription basis, with payment made once per year. It employs tiered pricing, where different levels provide access to specific modules. For instance, supplier management becomes available starting from the second tier, and one premium integration is exclusively accessible at the highest tier of the QMS pricing structure.
Some features can only be purchased as add-ons, such as document gap assessment and QMS audit.
Pricing tiers are determined based on the selected plan and the number of users. The system offers read-only and named user licenses. However, multiple-user licenses are not offered.
The system provides API integration with a wide range of popular apps, including Google Suite, Asana, Jira, Azure DevOps, Salesforce, TestRail, and more, allowing users to work with familiar tools. However, it does not integrate with Microsoft Office document editing applications.
Based on the reviews on customer reviews, Qualio is best known for its ease of use and user-friendly interface, being a quick and effective method of managing quality procedures.
It is also praised for its easy implementation and customer service.
What Are the Key Considerations Working With Qualio?
Key considerations for working with the Qualio solution are the following.
Qualio provides a native web-based document editor, which serves as a replacement for Microsoft Office for basic document editing tasks. However, it lacks the advanced features found in Microsoft Office. If you prefer to continue working within Microsoft Office, this solution may not be the most suitable.
Qualio necessitates that users undertake part of the validation activities. If you prefer not to invest additional effort and resources in validation activities, this solution might not be the best fit for your company.
Qualio offers only named and read-only licenses. This may not be the ideal option for teams that require multiple-user or shared licenses.
Qualio provides integration with a range of popular applications, including Google Suite, Asana, Jira, Azure DevOps, Salesforce, and others. This capability is particularly advantageous for teams already relying on these platforms for their operational needs.
MasterControl
Deployment: Web, iOS, Android, Windows
MasterControl provides manufacturing and quality management software.
The QMS software is a well-known solution for managing quality processes for various industries, including pharmaceuticals, medical devices, biotechnology, food and beverage, dietary supplements, and blood, biologics, and tissues.
MasterControl provides a solution mainly tailored to meet the needs of large enterprises.
Some of the MasterControl software’s features include document control, training management, nonconformance and deviation management, change control, equipment management, and audit management.
MasterControl has developed document control and quality management solutions compliant with FDA 21 CFR Part 11 regulation. Furthermore, the system supports compliance with several FDA regulations, ISO requirements, CLIA, EU MDR, and more.
The system is validated according to ISPE GAMP 5 and includes software validation with a patented Validation Excellence Tool (VxT). The VxT uses a risk-based approach to help users identify high-risk features or functions requiring additional usage testing. You can get VxT with an annual or quarterly subscription.
Subscriptions in MasterControl include validation and selected QMS modules depending on the plan acquired. The pricing is based on the subscription plan, number of users, and additional services.
The software only offers named user licenses with full access or read-only options.
MasterControl provides a six-phase implementation process: plan, discover, configure, validate, deploy, and onboard. Users are oriented in the process by the implementation team to ensure the efficiency and precision of the implementation.
The software integrates nicely with many major software applications typically used by companies, such as ERP, CRM, laboratory information management systems (LIMS), manufacturing execution systems (MES), and product data management (PDM) systems. It offers custom integration services to tailor the connection to user needs through API integrations.
Based on the customer reviews, MasterControl has good document management capabilities. Many reviewers praised the document management module’s ease of use and integration.
Additionally, reviewers appreciated the ability to link documents to other modules, such as nonconformances and CAPAs.
What Are the Key Considerations Working With MasterControl?
Key considerations for working with the MasterControl solution are presented below.
MasterControl is primarily positioned as an enterprise-level solution and is used by some of the largest companies in the world. Therefore, it might not be the best fit for small to mid-sized companies.
While MasterControl offers a robust suite of features to manage complex needs, its significant cost necessitates careful budget consideration before choosing.
MasterControl typically requires a time-consuming configuration process, which might be too resource-demanding for some organizations.
The software’s licensing model is limited to named user licenses, offering either full access or read-only functionality. If your company requires multi-user licenses, this option may not be a suitable fit.
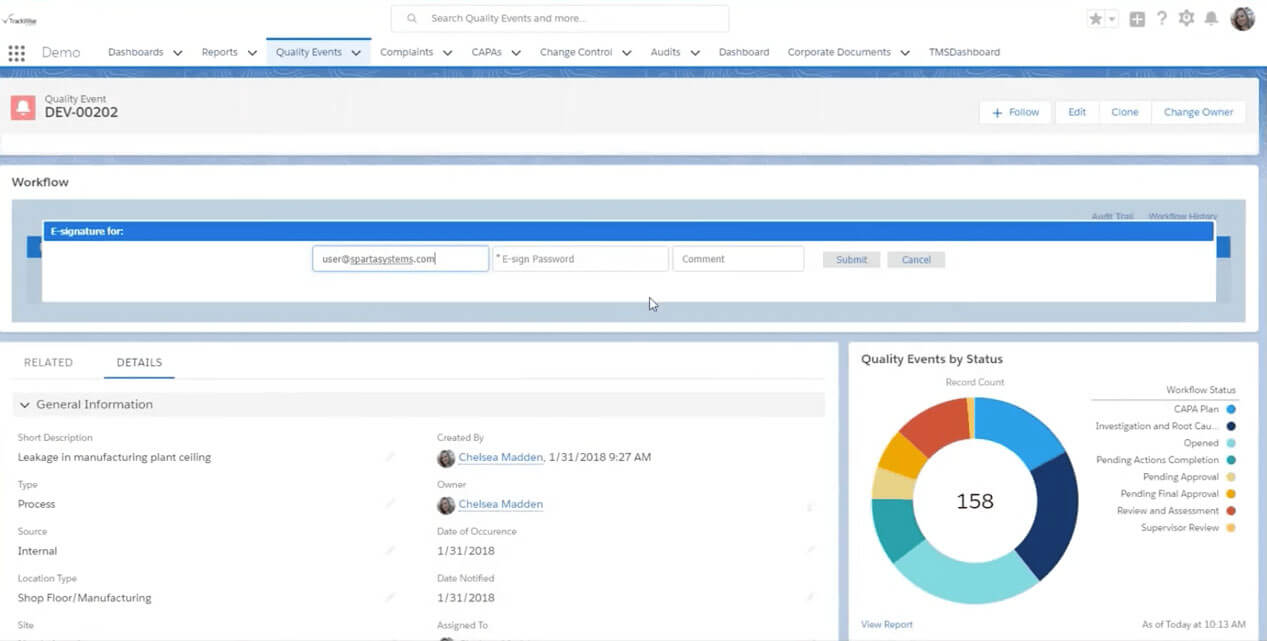
TrackWise
Deployment: Web, iOS, Android, Windows
TrackWise is offered in two formats: TrackWise and TrackWise Digital.
TrackWise is an on-premises QMS software, while TrackWise Digital is a cloud-based quality management software.
TrackWise Digital is also a quality management solution with AI-augmented decision-making capabilities, enabling a shift from reactive to proactive quality management. TrackWise Digital is built on the Salesforce platform.
Both software brings all quality processes together in a single place to give users an overview of compliance and operational effectiveness to increase efficiency, improve quality, achieve compliance, and reduce risk.
TrackWise offers a tailored software solution to industries, such as pharmaceuticals, biotechnology, medical devices, diagnostics, and food and beverage.
The software supports these companies’ QMS processes in document management, change management, nonconformance, deviation, training management, CAPA management, and more.
TrackWise offers online and onsite software training at the United States and Europe headquarters. Classes are provided for administrators and users.
The software supports users’ compliance with requirements, such as FDA 21 CFR Part 11, 211, and 820, EU Annex 11, MDR, ICH Q9, Q10, and Q11, GxP, and ISO standards.
The system is validated according to ISPE GAMP 5 and meets increased security standards in the Salesforce Cloud.
Specific pricing information for TrackWise is not readily accessible on the website, and potential customers need to contact their sales team for a personalized quote. The price appears to be influenced by factors such as the number of required user licenses, chosen quality processes, and implementation complexity.
Moreover, TrackWise is easy to integrate with other applications to streamline workflows and processes across all systems. TrackWise ensures real-time interoperability among product lifecycle management (PLM), ERP, CRM, LIMS, and MES systems.
Based on the customer reviews, TrackWise is good at being a highly configurable and customizable software platform. Users also appreciate integration with other business systems, such as Salesforce, making tracking and managing quality data easy.
What Are the Key Considerations Working With TrackWise?
Key considerations for working with the TrackWise solution are presented below.
TrackWise is an enterprise-level quality management software solution similar to MasterControl. Its complex features and potential cost structure might not align perfectly with the needs of small to mid-sized companies.
TrackWise offers extensive customization for companies with particular documentation needs. If your company needs highly personalized document management, TrackWise could be a suitable option. However, if you are looking for a system for a small or mid-sized company, the resources and cost required for customizing TrackWise might be too extensive.
While TrackWise offers a comprehensive suite of features, its premium pricing may make it more suitable for larger companies with substantial quality management needs.
Ideagen Quality Management
Deployment: Web, iOS, Android, Windows
Ideagen Quality Management, formerly Q-Pulse solution, is a modular eQMS software solution that supports companies to increase efficiency and enhance the accuracy of QMS processes.
Ideagen Quality Management is designed for several industries, such as life science, pharmaceuticals, manufacturing, healthcare, and food and beverage.
The software offers a complete suite of QMS modules, including document control, audit management, CAPA, reporting, supplier management, customer management, and more. The modules are integrated, and a workflow engine is used to auto-route notifications, reminders, escalations, approvals, and closures.
Ideagen Quality Management solution uses Microsoft Office applications for document editing. Their Collaboration Suite transforms Outlook and SharePoint into a secure hub of unified activity, collaboration, and transparency for regulated industries.
The software offers electronic signature ability, compliant with FDA 21 CFR Part 11 and EU GMP Annex 11 requirements. It also supports compliance with US and EU GMP regulations, ISO standards, and more.
The system is validated according to GAMP 5. However, full validation is provided to customers at an extra cost.
Ideagen works with users through the implementation process. This includes software deployment, product user documentation, and basic e-learning content.
Ideagen’s E-Learning content software provides training to employees free of charge in the basic version, which includes access to basic content, courses, and user reports. The professional version of E-Learning has an additional cost for more training features.
While specific pricing information is unavailable, users can purchase Ideagen Quality Management software as a perpetually licensed solution or a SaaS system.
Users can seamlessly integrate existing processes into the system and securely share data with external business systems via compliant APIs. The software integrates with ERP, CRM, LIMS, and many other systems.
Based on customer reviews, Ideagen Quality Management is good at document control and CAPA management workflows. Users appraise customer support as excellent, consistently exceeding responsiveness and resolution time expectations.
What Are the Key Considerations Working With Ideagen Quality Management?
Key considerations for working with the Ideagen Quality Management solution are the following.
Companies seeking comprehensive training support may not find the free basic E-Learning content sufficient due to limited features compared to the paid professional version.
Ideagen Quality Management offers a validation service at an additional cost. This may not be the best choice for companies seeking built-in validation or those with limited resources for managing their own.
Ideagen Quality Management is a modular eQMS software, which means different QMS modules are available in different plan tiers. While this flexibility caters to diverse needs, additional modules beyond the base tier may necessitate plan upgrades, resulting in increased costs.
Some customers have reported that they find Ideagen Quality Management to have a lack of integration between the different modules/features.
Greenlight Guru
Deployment: Web
Greenlight Guru is a cloud-based QMS software designed to improve speed and quality while lowering risk and cost. The system is well known for being designed for the MedTech industry.
The software provides comprehensive QMS modules, such as document management, training management, CAPA management, risk management, and more. It offers robust capabilities for requirements management and traceability matrix generation.
Greenlight Guru also offers specific MedTech and clinical modules, for example, design control and electronic patient-reported outcomes.
The software offers a native document editing feature, simplifying document workflows with in-app editing.
The QMS software aligns with 21 CFR Part 11 and 820, ISO 14971:2019, ISO 13485:2016, and FDA requirements.
With Greenlight Guru, users receive a complete validation package with every major software update. Software validation is an automated risk-based approach aligned with the FDA’s Computer Software Assurance (CSA) guidance and best practices of standards like ISO/TR 80002-2:2017 and ISO 13485:2016.
The pricing model is plan-based, offering three annual subscriptions: Essentials, Plus, and Professional. Each tier delivers a specific set of QMS modules and features at varying prices.
All plans include a dedicated customer success manager, validation package with each product update, online support, and on-demand academy, workshops, community, and online help resources.
Users are paired with a dedicated medical device industry expert to guide the implementation process, which can last from 2 to 8 weeks.
Greenlight Guru connects QMS data with external systems, such as ERP, CRM, PLM, Jira, or another application. Users can easily generate API keys and requests with an easy-to-use developer interface.
Based on the reviews on Capterra, Greenlight Guru is particularly recognized for its excellence in two key areas: design control and risk management. Reviewers consistently highlight the software’s intuitive interface and guided workflows that effectively streamline these complex processes, making them accessible even for users unfamiliar with quality management systems.
What Are the Key Considerations Working With Greenlight Guru?
Key considerations for working with the Greenlight Guru solution are the following.
Greenlight Guru is a suitable choice for MedTech companies seeking robust requirements management and traceability matrix capabilities. However, some users have reported that Greenlight Guru provides less flexibility in managing the traceability matrix than might be needed in some cases.
Greenlight Guru provides subscription plans that include predefined sets of QMS modules. Users who need additional modules beyond their selected plan will have to upgrade, which may result in increased costs. Especially smaller companies might find Greenlight Guru to be too expensive for their budget.
Greenlight Guru provides a built-in document editing tool directly within its interface. However, for companies preferring an eQMS that integrates seamlessly with Microsoft Office, other solutions might be better suited.
Dot Compliance
Deployment: Web
Dot Compliance provides a pre-configured cloud-based QMS solution powered by the Salesforce.com platform.
The Dot Compliance suite includes an extensive set of off-the-shelf-ready eQMS and compliance processes, enabling customers to deploy quickly and cost-effectively. It has interconnected QMS modules, such as document management, training management, change control, audit management, CAPA, complaint management, and more.
The software also integrates with Microsoft Office365 and Google Docs for easy check-out, check-in, and parallel collaboration between document authors, reviewers, and approvers.
Dot Compliance provides an AI assistant, Dottie AI, that automatically scans vast amounts of text and diverse data, identifies correlations, and provides ongoing, up-to-date AI insights.
The software is fully compliant with 21 CFR part 11, EU Annex 11, and supports compliance with FDA regulations, GMP, EU MDR, ISO 9001:2015, 13485:2016, 14791:2019, 27001:2022, and more.
Regarding software validation, Dot Compliant helps users ensure compliance with GAMP 5 validation with a fully executed validation package included.
Dot Compliance offers consulting and implementation services with in-house eQMS expertise to help users from project inception through deployment and beyond.
The pricing model is plan-based and offers three plans: QMS Xpress, Compliance Xpand, and Enterprise Xact. Each plan has different features, QMS modules, and pricing. For example, equipment management and product lifecycle management are only available from the Compliance Xpand plan onwards.
Exclusive in the Enterprise Xact plan, users can add modules for customized and configured quality and compliance processes based on their unique requirements.
Dot Compliance offers a free trial to allow potential users to explore the software’s features and functionality, user interface, and workflow integration before committing to a purchase.
The system provides an open API for integrating other systems, leveraging machine learning technology, AI, and IoT-enabled integration.
Based on the customer reviews, Dot Compliance is best known for being a user-friendly and cost-effective eQMS solution for Life Sciences companies. Customers mention the integration between all modules, facilitating the connection between different activities, as a strong suite.
What Are the Key Considerations Working With Dot Compliance?
Key considerations for working with the Dot Compliance solution are presented below.
Dot Compliance software is not fully validated according to GAMP 5 guidelines, which implies that companies might need to complete parts of the validation process themselves. Therefore, it may not be the best choice for companies seeking a fully validated eQMS.
While Dot Compliance offers several pricing plans, each includes a limited number of modules. Companies seeking specific modules not included in their chosen plan may need to upgrade to a higher-tier plan, incurring additional costs.
Dot Compliance offers additional support to its customers with implementation services which may incur additional costs.
QT9
Deployment: Web
The QT9 is a cloud-based eQMS that provides a unified quality management solution for companies of any size. The QMS is a solution for different industries, including medical devices, pharmaceuticals, chemicals, manufacturing, food and beverage, cosmetics, and more.
The system has over 23 built-in QMS modules, including document control, risk management, audit management, employee training, change, control, preventive maintenance, customer feedback, and more.
The software supports compliance with several Life Science requirements, such as FDA 21 CFR Part 11, 211, and 820, ISO 9001:2015, 13485:2016, and 17025:2017, GxP, and more.
QT9 QMS is fully validated and provides IQ, OQ, and PQ validation reports included for free for every version upgrade.
While specific pricing information for QT9 is not readily available, the platform offers flexible customization through multiple modules. This modular approach makes QT9 a scalable solution for companies of all sizes, from large corporations to small startups.
QT9 offers unlimited support and access to unlimited training, free updates, help center topics, a training academy, and live training.
Users can take advantage of a 30-day free trial to experience the software features, assess its value, and address any concerns before making a financial commitment.
QT9 system integrates with users’ existing technology. However, QT9’s strong integration is with its own ERP system, enabling different departments to work together more effectively by providing a single source of truth for all quality-related data.
Based on information from customer reviews, QT9 QMS is known for its user-friendliness and scalability. It offers several features and functionalities that serve businesses of all sizes, making it a versatile solution for diverse needs.
What Are the Key Considerations Working With QT9?
Key considerations for working with the QT9 solution are presented below.
QT9 modular design allows companies to customize their software to their specific needs. However, as the number of modules increases, this flexibility can lead to higher costs for some companies.
While QT9 offers integration with various systems, its most robust integration is with its own ERP system. This might limit flexibility for users who rely on different ERP solutions.
Scilife
Deployment: Web
Scilife is a quality platform for Life Sciences. It helps companies streamline quality management, automate processes, and transform quality into a company’s brightest asset. Scilife applies to many Life Science industries, such as pharmaceuticals, medical devices, CRO, CMO, and more.
The software offers several QMS modules, including document management, change control management, equipment management, CAPA management, audit management, training management, and more.
Scilife also integrates with Microsoft Office, enabling users to edit documents using Word, Excel, or PowerPoint applications. Documents are automatically saved in the Scilife cloud upon clicking save in the desktop application.
The system complies with the FDA 21 CFR Part 11 regulation for electronic signatures. Scilife helps companies comply with several requirements, such as FDA regulations, ISO 13485:2016, ISO 14971:2017, ISO 15189:2022, GxP, EU Annex 11, and more.
The software is validated according to GAMP 5 on the Amazon Web Services (AWS) platform. Scilife takes care of 95% of the validation activity, saving the user from redundant tasks and preserving your valuable resources. It is still necessary to evaluate the suitability of Scilife’s software and identify additional efforts needed for a specific application and intended use.
Scilife offers a tiered pricing model with three distinct plans: Essential, Core, and Core+. Each plan provides access to different QMS modules and functionalities, catering to varying organizational needs and budgets. For instance, the change control and CAPA management modules are only included from the Core plan onward. The Advanced plan exclusively allows workflow roles and permissions management.
Scilife also offers a free plan with unlimited users, up to 20 documents, 5 training, and more.
All paid plans include cloud infrastructure, software updates, a GAMP 5 validation documentation package for all updates, and unlimited customer support.
Scilife offers 4 types of system users: Administrators, Managers, Regular Users, and Read-Only Users. The first 3 user types can edit and participate in the approval workflow, and the Read-Only User type can only read documents and mark them as read and understood.
The system’s public API allows users to integrate any other system with Scilife, such as ERP, CRM, statistical process control (SPC), and others.
Based on customer reviews, Scilife excels in providing an accessible QMS platform, offering scalability and affordability that make it a perfect fit for companies of all sizes. Additionally, users consistently praise Scilife’s excellent customer support, known for its responsiveness and helpfulness.
What Are the Key Considerations Working With Scilife?
Key considerations for working with the Scillife solution are the following.
While Scilife provides GAMP 5 validation, users may need to perform additional validation activities. This might not be ideal for companies seeking a fully validated system out of the box.
Scilife integration with Microsoft Office allows users to edit documents on familiar applications like Word, Excel, and PowerPoint, effortlessly saving them in the Scilife cloud.
Scilife’s tiered pricing structure assigns different module sets with each plan. Companies with unique requirements may find it necessary to move to a higher tier, resulting in potential cost implications.
What Is the Best QMS Software for the Pharmaceutical Industry?
Some of the best QMS software for the pharmaceutical industry are Qualio, MasterControl, Ideagen Quality Management, SimplerQMS, QT9, and Scilife.
These QMS software solutions offer pharmaceutical QMS software with capabilities essential for ensuring consistent quality and compliance within a pharmaceutical manufacturing environment.
Some of the key capabilities include integrated QMS modules, such as document management, training management, change management, supplier management, audit management, complaints, deviation, out-of-specification (OOS), CAPA management, risk management, and electronic batch records.
The best pharmaceutical QMS software solutions provide 21 CFR Part 11-compliant electronic signatures, ensuring the integrity, authenticity, and reliability of electronic records and signatures. Additionally, these systems should feature controlled printing capabilities, restricting document circulation.
QMS software for the pharmaceutical industry must support compliance with requirements such as ISO 9001:2015, FDA 21 CFR Part 210, 211, and 212, EudraLex Volume 4 GMP, ICH Q7, and Q10, among others.
Furthermore, such systems would typically provide integration options with systems, such as CRM, ERP, LIMS, PLM, MES, and more.
What Is the Best QMS Software for the Medical Device Industry?
Some of the best QMS software for the medical device industry are Greenlight Guru, Qualio, SimplerQMS, TrackWise, Scilife, and Dot Compliance.
These solutions provide comprehensive medical device QMS software with features and capabilities that help ensure high and uniform quality, compliance, and product safety throughout the entire medical device lifecycle.
Key capabilities include comprehensive QMS process support, encompassing modules such as document control, training management, change control, design control, supplier management, audit management, complaints, nonconformance management, CAPA management, risk management, requirements management, equipment management and calibration management, and product lifecycle management.
These software solutions provide 21 CFR Part 11 compliant electronic signatures, ensuring the authenticity, integrity, and confidentiality of electronic records.
Medical device QMS software should support medical device companies in achieving compliance with requirements, such as ISO 13485:2016, MDR, IVDR, 21 CFR Part 820, and others.
A computer system for managing medical device QMS must also comply with Computer System Validation (CSV) requirements, as specified in ISO 13485:2016, EU Annex 11, and 21 CFR Parts 11 and 820, to ensure that the system functions as intended.
Such eQMS solutions would typically offer integration options with several systems, such as ERP, MES, CRM, and PLM, among others.
How Is SimplerQMS Different Compared To Other QMS Software Solutions?
SimplerQMS differentiates itself from other QMS software solutions in 10 ways, as listed below.
1. Seamless Office 365 Integration
SimplerQMS seamlessly integrates with Microsoft Office, enabling simultaneous editing within its cloud platform. All changes are automatically recorded in the audit trail, minimizing errors and saving valuable time.
2. Ability to Use Your Existing Forms and Documents
SimplerQMS maintains seamless business operations using your existing Word and Excel forms and templates within the system. SimplerQMS effortlessly migrates both Office documents and any additional file types, eliminating the need for document rebuilding and ensuring continuity.
3. Fully Validated eQMS System
SimplerQMS is fully validated according to GAMP 5 in compliance with FDA 21 CFR Part 11, EU GMP Annex 11, and ISO 13485:2016. We share continuously updated validation evidence with each customer to ensure ongoing compliance, including comprehensive IQ, OQ, and PQ documentation.
4. Unlimited 24/7 Support
We offer comprehensive and responsive customer support at no additional cost, accessible 24/7 through various channels: email, phone, video calls, a dedicated support portal, and an extensive online knowledge base.
5. Integrated Life Science Modules
SimplerQMS offers all modules within its subscription, providing comprehensive QMS functionality without additional fees. With SimplerQMS, you can leverage integrated features like document control, electronic signatures, training management, change control, CAPA, product management, supplier management, and equipment management, which are all included in the base price.
6. Configurable Metadata and Reporting
SimplerQMS enables you to utilize our metadata infrastructure to support established best practice processes seamlessly and generate comprehensive data reports through built-in or customizable views.
7. Experts in Life Science Processes and Audits
We have participated in over 100 audits and inspections by regulatory bodies and customers, including the FDA, notified bodies, national authorities, and industry peers. We have demonstrated our unwavering commitment to compliance with requirements such as GxP, FDA 21 CFR Part 11 and 820, EU GMP Annex 11, ISO 9001:2015, ISO 13485:2016, ISO 27001:2022, GAMP 5, MDR, and MDSAP, amongst others.
8. No Charge for Onboarding
SimplerQMS subscription encompasses all essential services: onboarding, implementation, configuration, training, validation, cloud hosting, and ongoing support. Our customers have unlimited access to live online training sessions, on-demand training videos, and a dedicated sandbox training environment, all at no additional cost.
9. GxP Enterprise Grade Infrastructure and Services
SimplerQMS includes Single Sign-On (SSO) technology accessible across Windows, Mac, and mobile devices through Microsoft Entra ID (previously known as Azure AD) integration. Additionally, it leverages ISO 27001:2022 and SOC2/3-compliant Microsoft Azure hosting, ensuring data reliability and compliance through disaster recovery and 15-minute backups.
10. Wide Integration Support
SimplerQMS seamlessly integrates with diverse systems, including ERP, CRM, project management, and LIMS, through its modern API and plugins, facilitating streamlined workflows and enhanced data exchange.
SimplerQMS provides comprehensive QMS software for life sciences. Manage quality processes from a single platform with ease – increase efficiency and ensure compliance.
What Is Quality Management System (QMS) Software?
Quality Management System (QMS) software, also known as digital QMS or eQMS (Electronic Quality Management System), is a digital platform designed to manage, control, and improve quality processes within an organization.
Electronic QMS software helps ensure that products and services comply with industry standards, regulatory requirements, and customer requirements by integrating and streamlining various quality-related processes like document control, change management, training, nonconformance and deviation management, CAPA management, audit management, and more.
Why Have Quality Management System (QMS) Software?
Having Quality Management System (QMS) software is essential for organizations aiming to maintain high-quality standards in their products, services, and processes.
This software streamlines and automates quality management system processes, ensuring consistent compliance with applicable regulatory requirements, industry standards, and customer requirements.
It aids in identifying and addressing inefficiencies, reducing errors, and managing documentation effectively. Additionally, it facilitates continuous improvement through access to data analysis and feedback mechanisms.
What Type of Companies Is Quality Management Software For?
Quality management software is particularly beneficial for companies operating in highly regulated industries, where adherence to stringent quality standards and regulatory compliance is critical.
This includes sectors like pharmaceuticals, medical devices, biotechnology, and other Life Science industries. These companies face unique challenges in meeting the rigorous requirements set by regulatory bodies such as the FDA and EMA, as well as standard bodies like ISO.
The software is also suitable for a wide range of other industries, including automotive, aerospace, and food and beverage, where maintaining high-quality standards is essential for business success, customer satisfaction, and compliance with industry-specific requirements.
Who in the Company Uses QMS Software?
QMS Software is used by a company’s diverse range of professionals and departments, as listed below.
Quality Assurance Professionals: These individuals use QMS software to ensure product quality and compliance through audit management, quality metric tracking, and CAPA implementation.
Quality Control Professionals: These professionals use the QMS software to streamline product testing and inspection, ensuring quality by documenting results and tracking nonconformances.
Regulatory Affairs Professionals: This group keeps the company compliant by monitoring changes in requirements, aligning practices, and managing submissions and documentation.
Production Managers: These individuals use the QMS software to monitor and control production, optimize processes, and ensure consistent quality throughout manufacturing.
Product Development Teams: These teams leverage QMS software to manage design controls and validation throughout product design and development.
What Are the Common Features of QMS Software?
Below are examples of the common features of QMS software.
Document Control: Automates and streamlines document control activities, providing control over all quality and regulatory documentation with centralized and secure management.
Training Management: Streamlines the implementation of employee training plans, allows for tracking progress, helps assess effectiveness, and notifies users of training activities.
Change Control Management: Facilitates an organized process for implementing changes in the QMS effectively without compromising structure or compliance.
Design Control: Helps manage all the necessary processes related to product design and meets design control requirements, from planning to commercialization.
Nonconformance/Deviation Management: Allows to record, evaluate, analyze, and manage nonconformances and deviations throughout the processes, ensuring quick and effective handling of issues through systematic management.
CAPA Management: Facilitates the identification, investigation, and implementation of corrective and preventive actions (CAPAs), streamlining CAPA processes to resolve quality events at their root cause effectively.
Complaints Management: Streamlines the recording, tracking, managing, and resolution of customer complaints in a timely manner.
Form and Template Management: Assists in creating and managing forms and templates for consistent and compliant collection of information.
Audit Management: Simplifies audit processes by effectively planning, executing, and tracking internal and external audits, reducing the time and effort needed to pass audits successfully.
Quality KPIs: Helps track quality metrics through quality KPI reporting, supporting data-driven decisions, and improving quality management and compliance efforts.
Risk Management: Facilitates effective risk assessments and mitigation strategies to safeguard your operations and compliance.
Equipment Management: Monitors and helps manage equipment, equipment maintenance, and calibration tasks to ensure optimal usage and compliance.
Supplier Management: Helps manage supplier management processes, from initial qualification and selection through ongoing monitoring and performance measurement.
Controlled Printing: Ensures secure, compliant printing practices with advanced access controls and traceability features.
Electronic Signatures: Streamlines document approvals and verifications with legally binding electronic signatures, enhancing workflow efficiency and traceability.
All these QMS modules and capabilities are part of the SimplerQMS software solution.
How To Buy QMS Software?
Buying QMS Software can be a complex process. Proper planning and a thoughtful approach when choosing the right QMS software can lead to a successful implementation.
Below are the 8 main steps involved in buying QMS software.
- Gather Requirements: Perform a thorough assessment of the current quality management processes and define the specific features and functionalities needed from the software. This step also involves requesting information from the QMS software vendors.
- RFI (Request for Information): Request information from vendors to gather general information about their products, features, and pricing models.
- RFQ (Request for Quotation): Obtain specific pricing information for the desired features and implementation scope. This allows for cost comparisons and helps determine budget feasibility.
- RFP (Request for Proposal): Request detailed proposals from shortlisted vendors for a more in-depth evaluation. RFPs usually include information on functionality, implementation plans, timelines, support services, and references.
- Research and Compare Vendors: Utilize industry resources, online reviews, and vendor websites to research and compare different QMS software options. Use the research to understand the market landscape and identify potential solutions that align with your needs.
- Shortlist Vendors: Select a few vendors that best meet the defined requirements and budget based on the information acquired in the RFQs and RFPs.
- Conduct Demos: Schedule demos with shortlisted vendors to experience their software firsthand, providing valuable insight into functionality, user interface, and overall suitability.
- Get a Trial: Consider requesting a free trial to test the software, evaluate its integration capabilities, and fit with the company’s current workflows and processes, and ease of use.
- Select Software: Evaluate shortlisted software based on demos, trials, proposals, and reference checks. Choose the software that best aligns with your needs, budget, and long-term vision.
- Negotiate: Negotiate the price, contract terms, and implementation details with your chosen vendor.
- Make Final Decision: Finalize the decision and purchase the QMS software.
To facilitate your decision-making process, download our free QMS Software Comparison Template.
The template enables you to compare various QMS software solutions side-by-side, evaluating each vendor’s features, functionalities, and pricing structures against your specific requirements.
How Much Does QMS Software Cost?
Most QMS software prices start raging from 10,000 USD to 20,000 USD per year.
Most QMS software solutions utilize a subscription-based pricing model, where companies pay a recurring fee for continued access and use. Subscription periods can be monthly, quarterly, annual, or multi-year.
The cost of QMS software can vary significantly depending on several factors, primarily the number of users, the number of QMS modules and features included, and the chosen license type.
To learn more about the pricing of SimplerQMS and explore the comprehensive range of features and services included, please visit our dedicated pricing page.
What Are the Different QMS Software Pricing Models?
The different QMS software pricing models are listed below.
- Tiered Pricing: The tiered pricing model offers different pricing tiers with varying QMS modules and feature sets. It allows companies to choose the tier that best aligns with their specific needs and budget. While many QMS solutions limit features by pricing tier, SimplerQMS delivers all modules and functionalities in one subscription price.
- Flat Rate Pricing: This pricing model offers a fixed monthly, quarterly, or annual fee for access to the system, regardless of usage or number of users. The simple and predictable cost structure is ideal for small companies with stable usage needs.
- Usage-Based Pricing: In this model, companies pay only for what they use, typically based on metrics like document storage, user actions, or integrations. It scales with the company’s growth, promoting efficient resource utilization. However, it is prone to unexpected spikes in costs during periods of high activity.
- Per User Licensing: This model charges a fixed fee per user per month or year. It is cost-effective for smaller companies with limited users but can become expensive as the number of users increases.
- Pay-Per-Module or Feature: The pay-per-module model allows companies to choose and pay only for the modules or features they need.
- Custom Pricing: Vendors offer customized pricing based on specific needs and user volume for complex enterprise-level solutions. This approach provides tailored solutions but requires negotiation and can be the most expensive option.
What Are the Different QMS Software License Models?
Below are examples of different QMS software license models.
- Named/Single-User License: Grants access to the QMS software to a single individual identified by name, ensuring accountability and control. This model is ideal when personalized accounts and individual user tracking are important.
- Concurrent/Multi/Shared License: Allows a designated or unlimited number of users to access the software using the same license. This cost-effective option suits companies with fluctuating user activity but requires careful management to avoid exceeding the concurrent user limit.
- Read and Sign/Light/Limited-User License: This provides limited access to the QMS software, such as for reading and electronically signing documents. This model is suitable for users who do not need extensive interaction with the system or only need to complete simple tasks.
- Perpetual License: Allows users to pay a one-time fee for indefinite use of the software. This model is ideal for those preferring a long-term solution without ongoing subscription costs.
- Site License: Grants access to software for use at a specific physical location or within a designated organizational unit. This model is ideal for organizations needing widespread software access at a single site or department.
- External/Guest-User License: Provides limited software access to individuals outside the primary user organization, such as contractors or partners. This model is suitable for organizations needing to grant system access to external stakeholders for collaboration or limited use.
How To Build a Business Case for eQMS?
Building a business case for implementing an eQMS involves the following aspects.
- Calculate the current QMS cost: Establish the baseline cost of existing quality management processes. The baseline serves as a benchmark to compare against potential savings offered by different eQMS vendors. Include the cost of employees involved in quality activities as well.
- Identify eQMS-generated savings: For each area in the company, identify the cost-saving value that implementing an eQMS will bring. Include all quality processes, such as nonconformance management, complaints, CAPAs, supplier management, employee training, audit management, and more. Usually, QMS software business case templates are filled with the cost-saving percentages for each QMS process.
- Evaluate software implementation savings: Calculate the cost savings by implementing comprehensive software. For instance, a fully validated software will save hours of work on validation efforts. Also, selecting a vendor that provides complimentary employee training will save costs on training activities on the company’s part.
- Consider savings from ongoing services: Check the ongoing services the QMS software vendor provides that are complimentary to the subscription price. Fully validated software, for instance, handles all validation activities, which means the company does not have to spend extra money on it. Additionally, systems compliant with 21 CFR Part 11 save audit consultants’ work hours.
- Prepare an overview of savings: Prepare a document to demonstrate the significant reduction in expenses achievable through eQMS implementation. Include the total cost of ownership related to the eQMS and the accumulated savings for the next 5 years.
To assess how an investment in an eQMS solution could benefit your company, download our eQMS Business Case template.
With the template, you can learn the specific benefits that SimplerQMS can offer your business, such as potential efficiency gains, cost savings, and compliance improvements. By clearly and concisely presenting this information to management, you can build a strong case for implementing an eQMS.
How Is QMS Software Implemented?
QMS software implementation is executed through a phased, structured approach.
Below is an example of how SimplerQMS employs a flexible process for implementation, organized into distinct phases. Each phase contains the deployment of specific QMS modules. The sequence of modules can be customized to meet the unique operational needs of each customer.
For instance, companies focused on manufacturing might prioritize and first implement Equipment or Suppliers Management modules. In comparison, other companies dealing with recorded issues might first prioritize the implementation of Nonconformances or Corrective and Preventive Actions (CAPA) Management modules.
In any instance, implementation phases have clearly defined milestones for each phase conclusion.
The SimplerQMS implementation process can be completed within 5-6 weeks. The time to implement the system is planned together with the customer and depends on the resources available and the number of documents ready for migration or in need of creation.
In kick-off meetings, the SimplerQMS Customer Success team collaborates with customers to outline a plan for the system implementation. SimplerQMS is flexible in incorporating incoming audits, inspections, and implementation deadlines, creating customized implementation plans that adapt to unique customer needs.
The SimplerQMS implementation phases are the following.
- Phase 1: Phase 1 includes system kick-off, software integration, system configuration, and training. In Phase 1, user licenses are assigned, and the system is integrated into the customer’s existing software ecosystem. Training is usually related to QMS core modules of Document Control, Training Management, and Change Control modules. Upon completion of training for each phase, there is the correspondent go-live process.
- Phase 2: Phase 2 usually consists of training for QMS modules related to Supplier and Vendor Management, Equipment Management, Design Control, Product Management, and Risk Management. Different modules can be implemented in each phase depending on customer needs and priorities.
- Phase 3: Phase 3 involves completing the training process for the remaining QMS modules, usually those modules include Customer and Feedback Management, Complaints, Nonconformance, Supplier Issues, Audit Management, and CAPA Management. In Phase 3, all implementation records are stored for compliance purposes, and the entire system is officially launched. The software becomes fully operational and accessible for real-world use, marking the culmination of the implementation process.
Throughout each phase of the implementation, the SimplerQMS Customer Success team guides and supports the customers to ensure a high-quality implementation and extensive training of all users.
The SimplerQMS Customer Success team provides ongoing support, helping customers with issues and troubleshooting even after implementation.
Who Is Responsible for Quality Management Software Implementation?
The responsibility for implementing quality management software falls on multiple employees within a company, reflecting a collaborative effort of the Quality Assurance (QA) team, IT department, project manager, and top management, among others.
In SimplerQMS, customers are in control of the project plan and the pace of work while we provide expert guidance and support throughout their QMS implementation process.
Our dedicated Customer Success team brings deep expertise and a commitment to success, providing personalized support, strategic counsel, and best practice recommendations.
When Should You Consider Implementing QMS Software?
You should consider implementing QMS software after planning and creating the documentation for your quality management system or be in the process of doing so.
Although knowing the exact time to implement a quality management system software solution is complex, below are some key indicators.
- Inefficient and Disorganized Processes: Relying on multiple spreadsheets or paper documents to manage quality processes creates confusion and potential errors.
- Communication and Collaboration Challenges: Limited communication and collaboration between quality teams and other departments like production and sales can lead to missed opportunities and inefficiencies.
- Lack of Visibility: Limited access to real-time quality data makes it difficult to make informed decisions, identify trends, and proactively address potential issues.
- Compliance Concerns: Difficulty meeting regulatory requirements due to manual processes and fragmented data.
What Is a Validated QMS Software?
A validated QMS software is an electronic QMS solution that has undergone a validation process to ensure it is fit for its intended use.
The QMS software validation involves analyzing system performance through test scripts. This process is performed according to the Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) requirements.
What Is the Difference Between Pre-Validated and Fully Validated QMS Software?
A pre-validated QMS software is a quality management system solution that has undergone a partial validation process, covering specific core functionalities and features.
A fully validated QMS software is a quality management system solution that has undergone a comprehensive validation process encompassing all its functionalities, configurations, and integrations.
A fully validated QMS solution reduces the costs for smaller companies by reducing the need to spend extra resources on verification.
SimplerQMS offers a fully validated eQMS, according to GAMP 5. We handle the entire validation process for you, ensuring your eQMS is compliant and ready to use from the moment you implement it.
What Are the Common Integration Options With QMS Software?
The common integration options with QMS software include but are not limited to the following.
- Enterprise Resource Planning (ERP): ERP software manages core business processes like finance, accounting, inventory, and customer relationship management.
- Customer Relationship Management (CRM): CRM captures customer feedback for quality improvement initiatives.
- Product Lifecycle Management (PLM): PLM software manages the entire product lifecycle from design to end-of-life, including design data, change orders, and product specifications.
- Manufacturing Execution System (MES): MES software manages and monitors the production process, controls equipment, tracks materials, and collects data.
- Laboratory Information Management System (LIMS): LIMS manages laboratory data and workflows, including test results, instrument calibration, and sample tracking.
SimplerQMS offers multiple integration options, including APIs, ETL (Extract, Transform, Load) import and export, plugins, and middleware connectors. These options allow integration with many software systems, such as ERP, CRM, CAD, CAM, LIMS, MES, PLM, and more.
What Are the Common Alternatives To QMS Software?
The common alternatives to QMS software are explained below.
- Document Management System (DMS): A standalone document management solution concentrates mainly on managing, organizing, storing, and tracking documents. Unlike an electronic QMS, a DMS does not offer comprehensive quality management process support. It lacks a suite of QMS modules and support for regulatory compliance related to the processes, which are essential features of a robust eQMS solution.
- Paper-based QMS: The system depends largely on physical documents and manual processes for managing quality within an organization. While it can be adequate for simpler processes, it presents several challenges. It tends to be labor-intensive, more costly, more prone to errors and can impede efforts in traceability and compliance due to its reliance on manual methods.
- Hybrid QMS: This system combines elements of paper-based and digital solutions to manage a company’s quality processes. It includes digital solutions for document management and basic workflow automation alongside some paper-based processes. This approach attempts to balance the traditional and modern methods. However, fully electronic QMS offers much superior efficiency and compliance capabilities.
Learn More About SimplerQMS Today
Now that you know more about the best QMS software on the market, it’s time to evaluate which one is right for your life science company. Let’s take the next step in streamlining your quality management journey today.