Solutions by Process
Regulatory Compliance Solutions
Ensure compliance with applicable regulatory requirements and accelerate your regulatory submission processes.
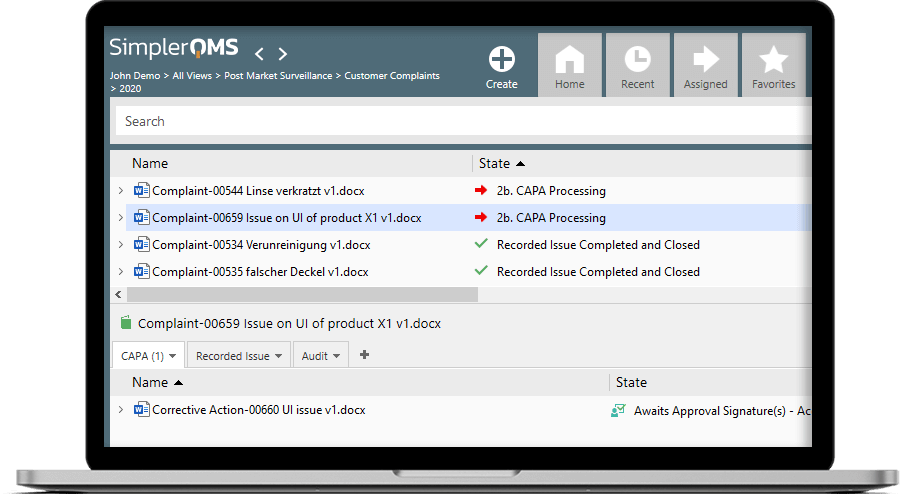
How Can SimplerQMS Help You Stay Compliant?
Regulatory compliance is no easy task and requires attention to detail and reliable strategy.
SimplerQMS’s regulatory compliance solutions help companies achieve compliance with Life Science requirements, including GxP, FDA 21 CFR Part 11, 210, 211, and 820, ISO 13485:2016, ICH Q10, EU MDR and IVDR, EU GMP Annex 11, ISO 9001:2015, and much more.
You can use our pre-configured workflows and forms, templates, and procedures, developed explicitly for Life Science companies to make regulatory compliance just a little simpler.
Audit Management
Document Control
Quality Management
Electronic Signatures
Form Management
Interested in Other Modules?
Regardless of the stage of your company, our QMS software modules cover all your needs.
Training Management
CAPA Management
Risk Management
Ready to Learn More?
To learn how you can make the most of SimplerQMS, request a free, personalized demo presentation.
