Good Manufacturing Practices (GMP) is a part of the Quality Management System that helps ensure products are produced and controlled according to quality requirements.
Companies must ensure that all procedures impacting the product’s identity, strength, quality, purity, and safety are validated and documented in order to comply with GMP regulations.
The specific GMP requirements may vary depending on the industry and product class. For instance, sterile pharmaceuticals have other specific requirements besides the basic GMP for medicinal products.
A Quality Management System (QMS) needs to reflect the requirements specified in the applicable GMP regulations so that companies can ensure high and uniform product quality.
In this article, we will discuss what GMP is, its importance and origin, some of the relevant regulations, and the role of an eQMS in GMP compliance.
Over the last decade, life science companies have been increasingly adopting electronic QMS (eQMS) to manage their quality processes more effectively than paper-based and hybrid systems.
SimplerQMS provides eQMS software designed for the life science industry. We helped several companies transition from traditional systems to eQMS and more easily manage all quality management processes in one system.
To learn more about how SimplerQMS can support life science QMS processes as per GMP requirements, book a personalized demo and talk to our experts.
In this article, you will learn the following:
- Defining GMP
- Origin of GMP Requirements
- Why Are GMP Regulations So Important?
- Major GMP Regulatory Requirements
- Role of an eQMS in Supporting Compliance With GMP Regulations
Defining GMP
Good Manufacturing Practices (GMP) are incorporated into the quality management system to help ensure companies manufacture their products with high and uniform quality according to controlled and documented processes.
Good practice requirements vary depending on the specific regulations of each industry.
The general acronym for Good Practice is GxP, where the letter “x” represents all the various processes where the good practice guidelines should be applied. For example, Good Manufacturing Practices are abbreviated as GMP, Good Laboratory Practices as GLP, Good Distribution Practices as GDP, Good Clinical Practices as GCP, and so on.
Regarding GMP, the practices can be associated with manufacturing food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices.
From now on, this article will focus on life science GMP requirements, especially for the pharmaceutical industry.
The GMP is important for pharmaceutical companies since manufacturing involves the complete product lifecycle, from raw materials and components to the finished product, including its discontinuation.
The GMP helps ensure that drug products are safe, reliable, and efficient for their intended use, complying with regulatory and customer requirements.
Companies must comply with the GMP or cGMP depending on the intended market. Companies to which GMP is applicable are constantly improving, and so are the related requirements. The most up-to-date requirements established are known as current GMP (cGMP).
Compliance with GMP requirements helps ensure the quality and safety of products. With cGMP, companies comply with the most current or updated requirements applicable to the industry.
To learn more about cGMP, you can read our article explaining what are Current Good Manufacturing Practices (cGMP).
Origin of GMP Requirements
Requirements to ensure the production of medicines that meet quality standards track back to 1906 in the United States with the creation of the Food and Drug Administration (FDA).
But it was in 1978 that the FDA established the requirements that became the cGMP in the 21 CFR Part 210 and 211 regulations as we know today.
In Europe, GMP requirements began in 1970 and are compiled in the EU GMP EudraLex volume 4. It is a comprehensive guide referring to the principles and guidelines for the GMP of medicinal products, which includes Directive 2003/94/EC for the principles of GMP regarding medicinal products for human use and investigational medicinal products for human use.
The EU GMP also includes some guidelines from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). For instance, guidelines Q7 (GMP guide for active pharmaceutical ingredients), Q9 (Quality Risk Management), and Q10 (Pharmaceutical Quality System).
Internationally, the World Health Organization (WHO) published the first draft of GMP guidance in 1968. In 1969, it was acknowledged as an integral part of the quality assurance of pharmaceutical products. More than 100 countries participating in the WHO Certification Scheme can use WHO GMP.
As GMP regulations were created and updated throughout history, pharmaceutical companies needed to control their processes accordingly.
For example, it became imperative to follow standard procedures, test batch samples, keep equipment calibrated, resolve deviations in a timely manner, train personnel, and so on.
A QMS that reflects a company’s specific needs is essential to achieve compliance with GMP requirements.
For instance, if a pharmaceutical company manufactures sterile drugs, there must be processes to control the number of particles in the air of specific areas at rest and in operation. QMS would include the processes and procedures in compliance with the applicable regulations, such as documenting particle testing, training staff to perform control testing, and keeping filtration equipment maintenance logs.
Why Are GMP Regulations So Important?
GMP regulations are essential to ensure manufacturing processes are controlled and well-documented to provide drug products made in accordance with validated procedures.
Compliance with GMP regulations is also important for the following reasons:
Handling Manufacturing Processes
Life science companies need to have well-documented procedures and control of processes. Having everything well described and organized helps ensure the manufacturing process is consistent and produces a uniform, high-quality drug product.
Protecting Products From Adulteration
Drug product batches need to be tested using appropriate samples to check if the established processes can ensure batch uniformity and integrity of drug products. Any deviations from the written procedures must be recorded and justified.
Reducing Costs
Safe and consistent processes help prevent product contamination, deviation, complaints, and recalls. Reducing these quality issues helps pharmaceutical companies avoid rework, changes in processes, and related financial costs.
Improving Customer Satisfaction
GMP provides manufacturing requirements to help ensure drug products meet the expected customer’s quality standards.
Providing safe, reliable, and efficient drug products improves the trust in the company by its customers and helps gain a competitive edge.
Driving Continuous Improvement
Companies must investigate and review complaints, recalls, and returned drug products. These reports can be used for evaluating the quality of each drug product to determine the need for changes in manufacturing processes.
Pharmaceutical companies with products under the GMP requirements must document several manufacturing process elements, such as master production, batch production, and control records.
An efficient eQMS helps manage all quality processes and the high volume of data and documents created throughout the product lifecycle. It also supports companies in achieving compliance with GMP requirements regarding document management.
An eQMS solution, such as SimplerQMS, provides life science companies with a cloud-based system to manage all documentation in one place. You can easily create, link, review, and approve documents while the software automatically records all actions in a document history view for a complete, time-stamped audit trail.
For more information, you can read our article on Quality Management System (QMS) documentation.
Major GMP Regulatory Requirements
Furthermore, we will discuss some of the main regulations and guidelines currently in force by the WHO, the European Union, and the United States regarding GMP. As well as give examples of how eQMS software supports compliance with GMP.
NOTE
Depending on the market, there are specific GMP requirements in the life science industry. This article will briefly discuss some of them. Please always refer to the official GMP regulation applicable to your company for comprehensive information.
FDA 21 CFR Part 210
Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs; General.
The 21 CFR Part 210 regulation specifies the minimum cGMP for manufacturing, processing, packing, or holding a drug to ensure the product’s safety, quality, and purity characteristics.
Companies must perform activities according to validated written processes and procedures to achieve compliance with this regulation.
This also generates a high volume of documentation to handle.
SimplerQMS solution streamlines document control activities by automating specific tasks in the workflow. We offer preconfigured workflows based on life science requirements to help ensure everything is documented as per the requirements.
The system automates the creation of periodic tasks, email notifications, and reminders to help ensure all activities are completed in a timely manner. You can easily create document relations to maintain full traceability, and much more.
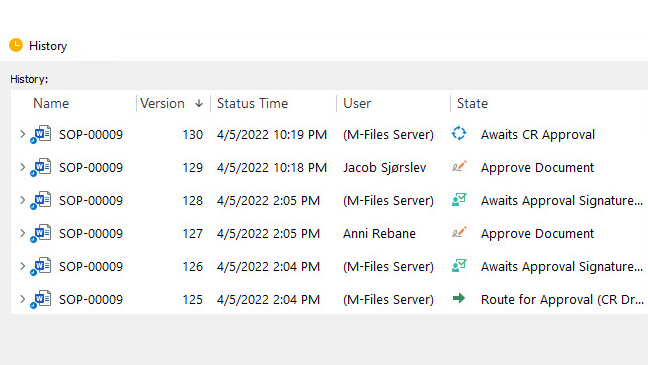
FDA 21 CFR Part 211
Current Good Manufacturing Practice for Finished Pharmaceuticals.
Regulation 21 CFR Part 211 establishes the minimum cGMP for the preparation of drug products for human or animal use.
This regulation includes requirements for personnel, facilities, equipment, labeling, packaging, distribution, deviations, contamination, reports, records, and more.
Among many requirements, pharmaceutical companies need to record and justify any deviation from the written procedures. This involves identifying and documenting deviations, implementing corrective and preventive actions, and recording all activities.
SimplerQMS provides an eQMS with built-in deviation management process handling. Companies can use document templates to create deviation documents, route documents to review, escalate deviations to a CAPA process, set up assignments, analyze data, and more.
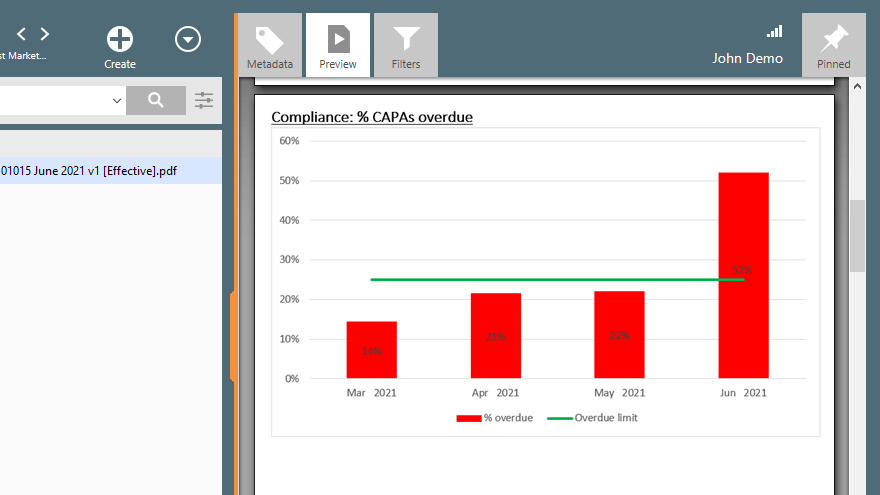
FDA 21 CFR Part 212
Current Good Manufacturing Practice for Positron Emission Tomography Drugs.
The 21 CFR Part 212 regulation specifies the minimum cGMP requirements for production, quality assurance, holding, or distributing Positron Emission Tomography (PET) drugs for human use.
One important GMP requirement for radioactive drugs concerns personnel. The quality of PET drugs is in part ensured by trained personnel with the experience to perform their assigned functions, following validated procedures.
Electronic QMS solution also allows companies to manage employee training more easily. Many features of the SimplerQMS training management module help companies achieve well-documented and compliant training processes.
It is possible to create training groups to include several employees in one training assignment. It is also possible to implement learning rules to automatically send notifications about retraining every time a relevant document is updated.
Companies can use quizzes to do training assessments and verify training effectiveness. This allows training effectiveness to be evaluated more accurately than just having trainees read and sign documents.
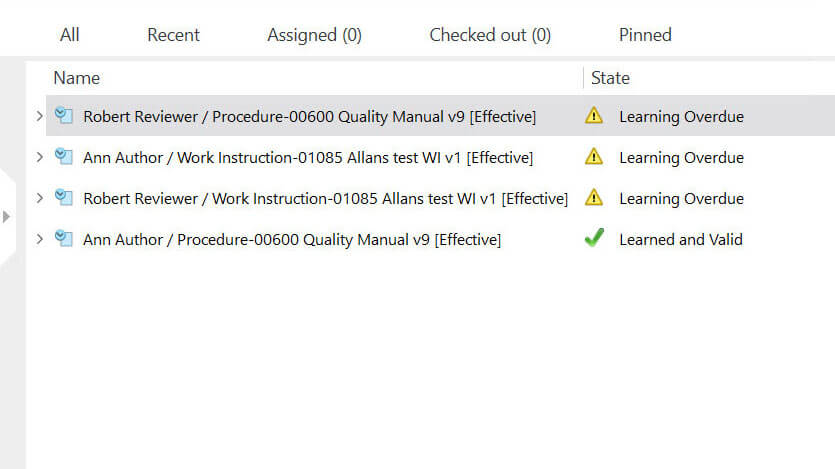
EU GMP EudraLex Volume 4
This is the fourth volume of “The Rules Governing Medicinal Products in the European Union.”
The EU GMP EudraLex volume 4 contains guidance for interpreting the GMP for medicinal products for human and veterinary use.
It includes other EU regulations to help companies achieve GMP compliance, such as Directive 2003/94/EC.
An important regulatory requirement is the need to conduct inspections. Companies must undergo external and internal audits and propose any necessary corrective measures in case of audit findings.
SimplerQMS simplifies the creation of audit plans for external, internal, and vendor audits through the quality audit management module. Various tasks can be automated, such as periodic task creation, email notifications, reminders, etc., to help decrease the number of manual tasks related to audits.
The system automatically records all activities in documents to create a time-stamped audit trail and provides powerful search functionality to facilitate document retrieval, for instance, during an audit situation.
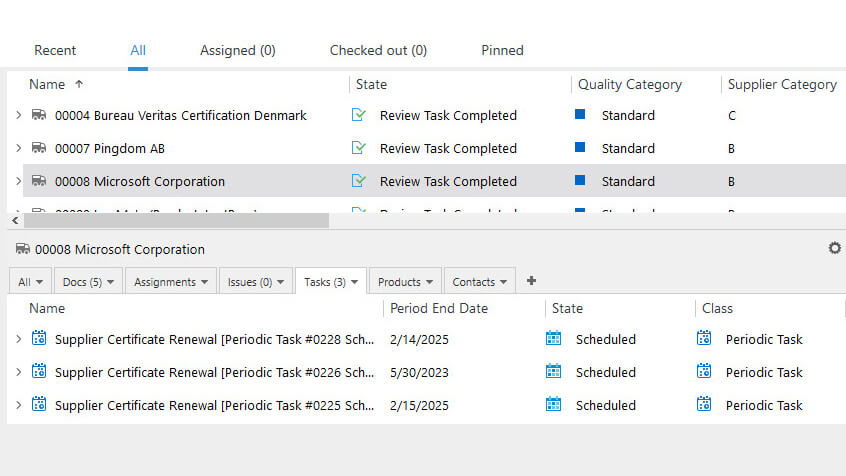
ICH Q7
GMP Guide for Active Pharmaceutical Ingredients.
The ICH Q7 is a guideline to provide guidance regarding GMP for manufacturing active pharmaceutical ingredients (API).
The ICH Q7 is currently implemented in several countries, such as the United States, Japan, Europe, Brazil, Mexico, Canada, Turkey, and more.
The guide covers many requirements regarding quality management, personnel, facilities, equipment, materials, laboratory controls, complaints, clinical trials, etc.
Among them, pharmaceutical companies must implement a formal change control system to manage all changes in documents and processes that may affect API production.
SimplerQMS offers a robust change control management module that streamlines change request workflow and supports companies’ continuous improvement processes.
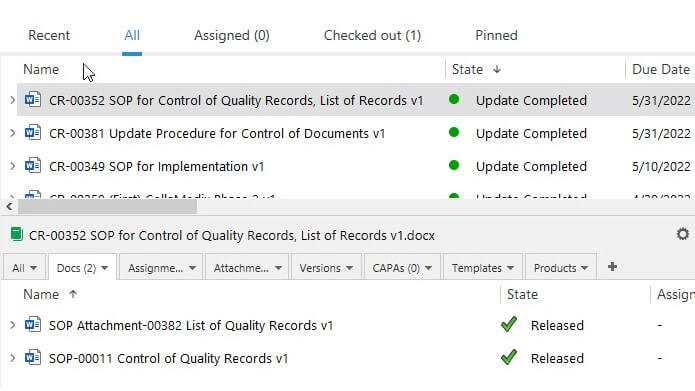
Companies can create change requests and easily link them to documents, equipment, and products. The software allows the selection of any number of people to participate in the change request workflow and automated document routing for review or approval.
Actions or periodic tasks can be scheduled to monitor the change’s impact and effectiveness and have the system send automatic reminders and notifications, allowing employees to keep track of change requests and their required activities.
ICH Q9
Quality Risk Management.
The ICH Q9 guideline provides principles and methodologies for quality risk management that can be applied to different aspects of pharmaceutical quality, including manufacturing.
Effective quality risk management helps ensure the quality of the drug product to the end-user by providing ways of identifying potential quality issues during manufacturing.
SimplerQMS helps companies make risk-based decisions by monitoring risk documentation status across all processes. Our risk management software module simplifies the creation of documents for risk assessment and analysis using customizable templates.
Companies can relate risk documents to drug products, equipment, customers, and vendors to ensure the traceability of information.
ICH Q10
Pharmaceutical Quality System
The requirements for one comprehensive model of a pharmaceutical quality system are specified in the ICH Q10 guideline.
The Q10 guideline covers the entire lifecycle of a product and is intended to be used together with regional GMP requirements. Having an effective pharmaceutical quality system in place drives sustainable GMP compliance.
SimplerQMS offers complete pharmaceutical QMS software with all QMS modules available in one solution, including document management, change control, employee training, deviations, CAPA management, and much more.
Our system is customizable and flexible to include all the necessary steps in your processes while supporting your company in achieving compliance.
WHO GMP
The WHO GMP guidelines for biological products are intended to guide national regulatory authorities and manufacturers of biological products.
This guideline covers requirements on the pharmaceutical quality system, risk management, personnel, equipment, quality control, documentation such as batch processing records, and more.
The WHO GMP requirements can be used as national requirements if a country desires, to adopt the guidelines entirely or with justified modifications.
Those are some of the relevant regulations regarding GMP.
Other regulations can apply to life science companies in the pharmaceutical industry, such as 21 CFR Part 314 (Applications for FDA Approval to Market a New Drug) or 21 CFR Part 600 (Biological Products).
Always make sure to verify the regulatory requirements applied to the country and intended market you want to operate in!
Role of an eQMS in Supporting Compliance With GMP Regulations
Companies can use traditional paper-based or hybrid QMS to comply with life science requirements. A manual QMS can be used successfully in small companies with adequate resources for handling the paperwork.
However, traditional formats of quality management systems have some challenges, such as the potential of losing documents and records, physical storage issues, unauthorized access to files, etc.
Also, manual processes can be tedious and difficult to track. Companies may find themselves facing difficulties when it comes to maintaining compliance with GMP regulations.
This is where an electronic QMS (eQMS) can help. Best eQMS software solutions offer the ability to automate many tasks associated with processes, such as document management, change control, employee training, and many more.
Implementing an eQMS system ensures that organizations meet all their quality management system requirements while supporting GMP compliance.
SimplerQMS provides an eQMS that is both secure and reliable. Our software provides broad process support, from document and change management, to risk management, employee training, vendor management, and more. It makes it much easier for organizations to maintain compliance with GMP regulations across all quality management processes.
With SimplerQMS, GMP documents and records can be created using customizable templates, and preconfigured workflows help ensure the process follows the necessary steps, and all necessary data is collected.
Furthermore, the system is fully validated according to ISPE GAMP5 and is re-validated upon the creation of a new version or upon applying standard updates. It is also compliant with FDA 21 CFR Part 11, which sets the guidelines for electronic signatures and electronic records, as well as EU GMP Annex 11, which provides good manufacturing guidelines for computerized systems.
All the above and many other features provide a software solution that makes GMP compliance easier, saving time and money in the process.
If you are unsure whether an investment in an eQMS is worth it for your company, then check out our eQMS Business Case template – which you can download below.
It can help you assess the value of an eQMS for your company and includes the necessary material for presenting your findings to the management.
Final Thoughts
Highly regulated life science industries, such as pharmaceutical and biotechnological companies, must comply with several regulations and standards depending on the intended market.
Among them, the GMP regulations help ensure medicinal products are produced and controlled according to quality requirements.
Having a QMS that reflects the applicable GMP requirements allows companies to achieve compliance and place safe, reliable, high, and uniform-quality drug products in the market. The QMS needs to have all the relevant processes and procedures in place to control the processes related to manufacturing drug products according to the GMP requirements.
Traditional paper-based and hybrid QMS can be used for small companies with adequate resources to do the manual work. However, implementing an eQMS can be instrumental in supporting GMP compliance, as it provides automation and streamlining of processes.
Book a personalized demo and talk to our experts if you want to learn more about how SimplerQMS can help streamline your quality management processes and support compliance with GMP regulations.