The deviation management process is a systematic approach to identifying, analyzing, solving, and documenting events that deviate from the established protocols during the development, manufacturing, and commercialization of products.
It is an important part of quality management in the pharmaceutical industry, as it helps to ensure the safety and quality of pharmaceutical products.
This article will discuss the definition of deviation, its different classification types and categorization, guidelines applicable to deviation management, and deviation management process flow. We will also explore the role of QMS software in effectively managing deviations.
SimplerQMS provides eQMS software tailored for pharmaceutical companies with robust deviation management capabilities. Schedule a demo today and talk to one of our quality consultants to get a more comprehensive understanding of our QMS software.
The following topics are further discussed in this article:
- What Is a Deviation?
- What Is Deviation Management?
- What Are the Different Classification Types of Deviation?
- How Are Deviations Categorized Based on Risk?
- What Are the Deviation Management Guidelines in the Pharmaceutical Industry?
- What Is a Deviation Management Process Flow?
- What Is the Role of Deviation Management Software in Streamlining the Deviation Management Process?
What Is a Deviation?
A deviation is any departure from approved processes, procedures, instructions, specifications, or established standards.
In the pharmaceutical industry, deviations can occur during drug product development, manufacturing, labeling, packing, sampling, testing, storage, distribution, and other processes.
The deviation definition can vary slightly depending on the regulatory authority and requirements governing the company’s processes.
Some definitions of deviations according to different regulatory organizations and requirements are as follows.
International Organization for Standardization (ISO):
According to ISO 9001:2015, deviations in the context of quality management systems refer to the positive or negative effect resulting from a divergence from the expected or established standards.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH):
In the ICH Q7 Good Manufacturing Practice (GMP) guide for active pharmaceutical ingredients, a deviation is defined as a departure from an approved instruction or an established standard.
Food and Drug Administration (FDA):
In the FDA’s guidance on quality management systems for the pharmaceutical industry and current Good Manufacturing Practice (cGMP) regulations, a deviation is described as a result that falls outside the expected range or fails to fulfill a specific requirement.
What Is Deviation Management?
Deviation management is the systematic process of identifying, assessing, and addressing any deviations that occur from approved instructions or established standards within a company.
The objective of deviation management in the pharmaceutical industry is to promptly identify, investigate, and resolve any deviations.
Deviation management is an essential QMS process in a Pharmaceutical Quality Management System.
By promptly identifying and addressing deviations, pharmaceutical companies can provide safe, uniform, high quality, and effective products to patients while maintaining their reputation in the market and compliance with regulatory requirements.
Effective deviation management plays a crucial role in handling diverse types of deviations, streamlining processes, and fostering continuous improvement.
What Are the Different Classification Types of Deviation?
There are two main classification types of deviations: planned and unplanned deviations.
Planned Deviation
Planned deviations refer to pre-approved and intentional deviations from standard procedures or processes.
These deviations are planned and justified in advance, serving various purposes, such as process improvement, method validation, and temporary process changes.
Some examples of planned deviations include:
- Implementing a temporary manufacturing process change to test and validate potential efficiency improvements.
- Using an alternative raw material as part of a trial to assess its suitability without affecting product quality.
- Temporarily adopting an alternative testing method to validate its accuracy and reliability compared to the standard method.
Change control and change requests are essential components of managing planned deviations in a controlled and systematic manner.
Change request documents are part of the change control process. They provide a formal and structured way to propose and justify the planned deviation, outlining its purpose, scope, and potential impact.
The change control process in the pharmaceutical industry ensures that relevant personnel can assess and approve the proposed change, considering its implications on product quality, safety, and regulatory compliance.
Implementing planned deviations through these processes helps maintain control, traceability, and well-structured documentation.
Unplanned Deviation
Unplanned deviations refer to a departure from approved procedures without prior notice or intention.
Various factors, such as equipment malfunction, employee error, environmental events, or others, can cause them.
Unplanned deviations can significantly impact product quality and safety, and such deviations should be investigated promptly to identify the root cause and prevent them from happening again.
Some examples of unplanned deviations include:
- The sudden breakdown of manufacturing equipment leads to deviations from standard operating conditions and affects product quality.
- Deviations are caused by unforeseen events like power outages, extreme weather conditions, or natural disasters that disrupt manufacturing.
- Accidental introduction of foreign materials into the production process, leading to material contamination and deviation from product specifications.
Companies employ Corrective and Preventive Action (CAPA) processes to effectively address and resolve unplanned deviations.
When an unplanned deviation occurs, the CAPA process is used to implement corrective actions, aiming to mitigate the immediate deviation impact. Afterward, preventive actions are developed to avoid the recurrence of similar deviations in the future.
The CAPA process implementation varies based on each specific company’s established procedures. Companies assess the severity and categorization of the deviation based on risk to determine whether the CAPA process will be initiated.
How Are Deviations Categorized Based on Risk?
Deviations are categorized based on risk levels to assess their potential impact on product quality, patient safety, and the regulatory requirements companies are subject to.
Companies can have different criteria for defining deviation categories, which are well-described in their protocols and procedures. The categorization of deviations may vary based on the nature of the product, industry requirements, and the company’s risk management needs.
The risk-based deviation categories usually include:
- Incidents
- Minor deviations
- Major deviations
- Critical deviations
Incident
An incident is an unplanned or uncontrolled event that does not directly affect the manufacturing process parameters or product quality.
Incidents are generally unrelated to GMP requirements and are often not considered deviations.
However, an incident affecting product specifications can be categorized as a cGMP-related nonconformance.
This categorization varies between companies and must be clearly specified in the company’s procedures.
Examples of incidents are listed below:
- Accidental material spillage occurred in the cleanroom.
- Personnel not appropriately dressed inside the production area.
- A minor typographical error in the documentation that does not affect the overall process or product outcome.
Minor
A minor deviation involves divergences from established procedures or standards that have a noticeable but limited impact on product quality or regulatory compliance.
Examples of minor deviations are listed below:
- A slight variation in the size or appearance of a product that does not affect its efficacy or safety.
- A deviation in the production timeline that leads to a slight delay but does not compromise the overall product quality.
- Documentation errors that do not compromise data integrity.
Major
A major deviation indicates a significant departure from established procedures or standards that can considerably impact pharmaceutical drug quality and regulatory requirements.
Examples of major deviations are presented below:
- A deviation in the manufacturing process results in an out-of-specification (OOS) product requiring investigation and corrective actions to prevent further occurrences.
- A failure of critical equipment during production leads to a significant delay in the manufacturing process and a potential impact on product quality.
Critical
A critical deviation denotes a severe nonconformance from established procedures or standards that poses meaningful risks to product quality, patient safety, or compliance with applicable requirements.
Examples of critical deviations are given below:
- Contamination of a critical raw material used in the production process that may lead to a serious health risk for patients.
- Issues in the packaging process that result in incorrect labeling or dosage instructions which can pose a high risk of incorrect medication intake and patient harm.
- The failure of the HEPA filters in the cleanroom that may lead to potential contamination risks and compromise the integrity of the controlled environment.
When an unexpected event occurs, pharmaceutical companies need to categorize the event to be able to follow the appropriate procedures to solve the issue.
An example of a decision pathway that can be used to categorize incidents and deviations is provided below.
The first step is to identify if the event has a significant impact on the manufacturing process parameters, SOPs, or cGMP. If the event does not have a significant impact, then the event is considered an incident.
However, it is considered a deviation if the event significantly impacts and affects a product attribute.
To determine the severity of the deviation, it is necessary to know if it affects the operations or critical parameters and equipment or instruments.
The deviation can be categorized as minor if none of those are affected. Otherwise, it can be considered a major or critical deviation.
The flowchart below illustrates the deviation categorization process.
What Are the Deviation Management Guidelines in the Pharmaceutical Industry?
Deviation management guidelines vary depending on the specific market that pharmaceutical companies operate in and their product.
The deviation management process must align with the applicable requirements and industry best practices.
In the pharmaceutical industry, various standards, regulations, and guidelines outline requirements regarding deviation management. Below are some of them.
NOTE
Since the list of standards, regulations, and guidelines provided below is not exhaustive, it is essential to consult the applicable requirements for your company for official information.
ISO 9001:2015
The ISO 9001:2015 is a general quality management system standard that specifies requirements for quality systems in several industries. Some pharmaceutical companies choose to comply with ISO 9001:2015 standards.
The standard addresses deviation management in Section 10.2. When a deviation occurs, companies must react to the problem by taking control of the situation and investigating the root cause to correct the problem and manage its consequences.
Preventive actions should be implemented to avoid the recurrence of the deviation, considering similar issues. Companies need to keep records of the deviations, actions taken, and the results of corrective actions.
FDA 21 CFR Part 211
The 21 CFR Part 211 outlines the cGMP requirements for finished pharmaceuticals for companies in FDA-regulated industries.
According to 21 CFR 211.100, pharmaceutical companies must have written procedures for production and process control deviations.
Deviation documents should be drafted, reviewed, and approved by relevant departments and the quality control unit. Procedures must be followed during production, and any deviation from them must be recorded and justified to maintain product integrity and compliance.
ICH Q7
The ICH Q7 defines the GMP guidelines for active pharmaceutical ingredients (API).
Within ICH Q7 quality management guidelines, Section 2.16 emphasizes the importance of documenting and explaining any deviation from established procedures.
The quality documentation related to deviations ensures transparency and accountability in the manufacturing process, helping to maintain the quality, safety, and consistency of API production.
EU and PIC/S GMP Guide Part 1
In the Eudralex GMP and PIC/S GMP Guide Part I for medicinal products, Section 1.8 (vii) specifies that significant deviations from established procedures must be thoroughly recorded.
These deviations should be investigated comprehensively to identify the root cause. Based on the findings, appropriate corrective and preventive actions must be implemented to prevent similar deviations from occurring in the future.
(EU) No 1252/2014
The European Union regulation 1252/2014 sets forth the principles and guidelines of GMP for active substances for medicinal products.
It is established in Article 7 that during the manufacturing process, all quality-related activities, including deviation management processes, must be documented as they are performed. So, if any deviation occurs from the written procedures, it must be documented and explained.
Furthermore, if any deviations impact the quality of the active substance or prevent it from meeting the specifications, a thorough investigation must take place. The findings and conclusions of the investigation must be documented.
There are common requirements across all deviation management regulations, standards, and guidelines that outline the typical steps in the deviation management process.
What Is a Deviation Management Process Flow?
The deviation management process flow follows a systematic approach to identifying, reporting, investigating, documenting, correcting, and preventing deviations from approved or established standards. It outlines how to handle deviations in a structured and organized manner.
In this section, we will discuss the steps involved in the deviation management process and how QMS software like SimplerQMS streamlines each step.
The deviation management process flow typically follows the following steps:
- Identification
- Reporting
- Investigation
- Documentation
- Implementation
Identification
The initial phase involves understanding the event that led to a deviation. Deviations can arise at any stage during the product manufacturing lifecycle.
As mentioned earlier, these events are classified as incident, minor, major, or critical, depending on their severity and potential impacts.
The correct categorization helps in prioritizing deviation resolution and determining the appropriate level of investigation and corrective actions required to address each type of deviation.
In SimplerQMS, categorizing deviation is a straightforward process.
Companies can create their own categories and select the relevant ones using dropdown menus when drafting deviation-related documents.
Reporting
The Deviation Report holds significant importance in the deviation management process. It is a formal document used to record and document each identified deviation.
The Deviation Report provides a comprehensive account of the deviation, including its nature, location, date, time, personnel involved, and potential impact on product quality or safety.
When a deviation occurs, the department responsible for the process where the deviation took place is usually responsible for filing the Deviation Report. Timeliness is essential in this phase, and the report should be filed within a specified timeframe.
For instance, when a Deviation is created in SimplerQMS, the system automatically sends reminders and notifications to responsible team members regarding their assigned Deviation Reporting tasks. Notification helps ensure employees are aware of their responsibilities and the due date to perform their tasks.
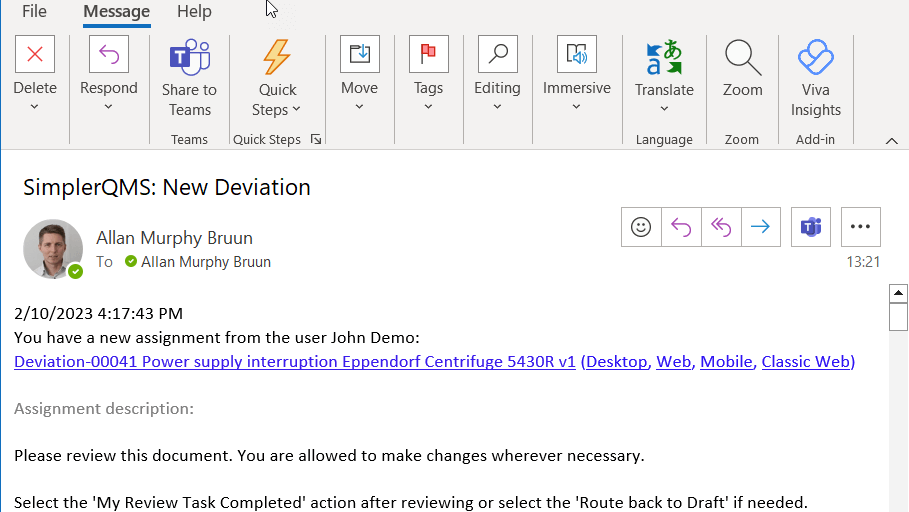
Investigation
After identifying and reporting the deviation, the next step is to conduct a thorough investigation to determine its root cause.
Root-cause analyses are particularly crucial for major or critical deviations, as they have a significant impact on product quality or compliance with regulatory requirements.
In cases where root-cause analysis is required, after completion, the investigation phase proceeds to assess the need for Corrective and Preventive Action (CAPA).
CAPAs are initiated depending on specific criteria established by the company, such as recurring issues and deviations that affect the overall product quality or regulatory compliance.
This systematic approach ensures appropriate actions are taken to address deviations, improve product quality, and maintain compliance with regulatory requirements.
SimplerQMS provides eQMS with an interconnected CAPA management module to address deviations and other issues if necessary. For instance, Issue Handlers can determine the need to escalate a deviation to a CAPA and link all documents to ensure traceability.
CAPA document is then created using a document template. Companies can use already provided templates by SimplerQMS and edit them as necessary or migrate their existing ones to the system.
Documentation
The deviation management process must be documented. This includes the documentation of the identification of the deviation, the investigation, the corrective action, and the preventive action. Deviation-related documents can be linked together to promote traceability of information.
An essential component of deviation management documentation is the audit trail, which maintains a chronological record of all actions and changes made during the process.
Audit trails provide transparency and accountability, as well as evidence that requirements have been met, facilitating efficient tracking of deviations throughout their lifecycle.
In SimplerQMS, deviation documents can be related to specific document versions using hyperlinks. This ensures that the actions performed are accurate and reflect the procedures and the specific versions at the time of the deviation.
Our system automatically records all actions performed in documents and generates a time-stamped audit trail. Companies can keep track of deviation-related documentation progress by monitoring document status in highly customizable views.
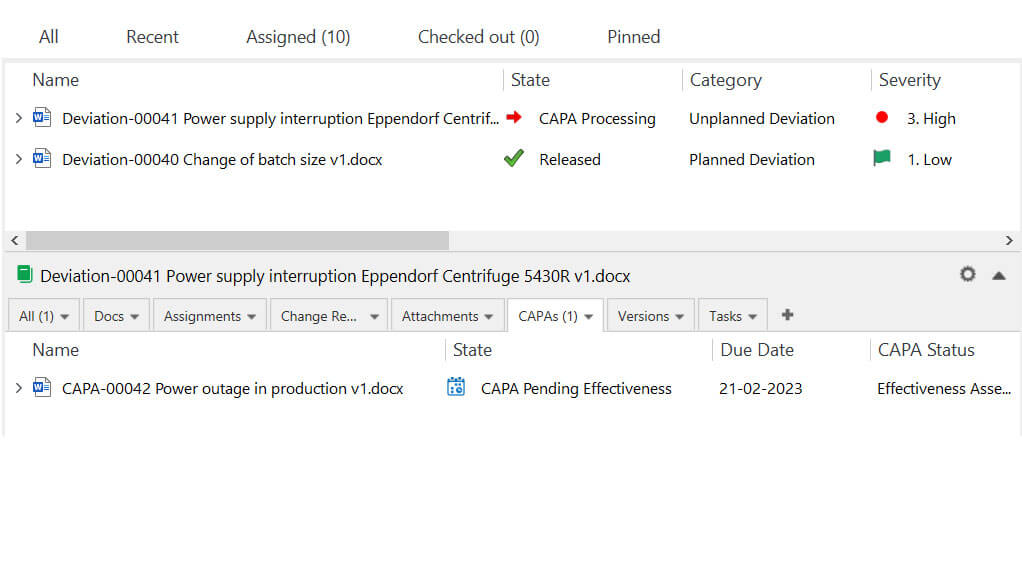
Implementation
During the implementation phase, corrective and preventive actions are put into effect to prevent the deviation from happening again in the future. Effectiveness checks should be conducted to ensure that the actions are having the desired effect.
Timely and effective implementation of these actions is essential to ensure continuous improvement and maintain product quality and compliance with regulatory requirements.
SimplerQMS enables companies to create and schedule effectiveness checks with just a few clicks. The system simplifies assigning responsible people and setting deadlines. Notifications and reminders are automatically sent to ensure effectiveness checks are performed on time.
What Is the Role of Deviation Management Software in Streamlining the Deviation Management Process?
Deviation management system software plays a critical role in streamlining the deviation management process. By automating many of the manual tasks involved in deviation management, the software can help companies identify, report, investigate, document, and resolve deviations more quickly and effectively.
SimplerQMS provides an eQMS software solution tailored for Life Science companies together with robust deviation management capabilities.
SimplerQMS enables users to easily create deviation documents using forms and templates. Pre-defined workflows guide users through the processes this way supporting compliance with the Life Science requirements. For example, after investigation, deviations can be directly escalated to the CAPA process while linking all relevant information together.
SimplerQMS supports compliance with several Life Science requirements, such as 21 CFR Part 211, 212, and 820, ISO 9001:2015, EU Volume 4 GMP Part I, ICH Q7, ICH Q10, ISO 13485:2016, MDR and IVDR, and more.
We help companies comply with regulatory requirements by providing comprehensive QMS process support inside the system.
Some of the QMS processes, besides deviation management, that the SimplerQMS solution supports include document management, change control, employee training, CAPA management, customer complaint management, audit management, supplier management, and more.
If you are considering an eQMS implementation, you can download our free eQMS Business Case template. The tool allows you to evaluate the economic benefits and time-saving potential that an eQMS brings and equips you to present robust findings to your management.
Final Thoughts
Deviation management is a systematic process used to identify, analyze, resolve, and record any events that differ from the standard protocols.
This systematic approach plays a vital role in maintaining the uniform and high quality of pharmaceutical products, ensuring that only safe and effective drugs reach the market.
Using manual paper-based or hybrid systems, some companies can manage deviations. However, leveraging QMS software with deviation management process support offers multiple advantages, making the entire deviation management process more efficient and streamlined.
SimplerQMS offers an eQMS software solution designed specifically for Life Science companies with robust deviation management capabilities.
Discover how SimplerQMS can help streamline your QMS processes. Book a personalized demo with our knowledgeable eQMS system experts today to experience its benefits firsthand.