Current Good Manufacturing Practices (cGMPs) are a set of regulations that aim to ensure the quality and safety of products manufactured for human and animal use.
The cGMPs are enforced by the Food and Drug Administration (FDA) and apply to companies operating in the United States.
They apply to a wide range of products, including drugs, biologics, medical devices, food, and dietary supplements. The specific requirements of cGMPs vary depending on the type of product.
In this article, we will discuss the importance and objectives of cGMPs, key requirements and areas, and how to maintain compliance with cGMPs. We will also discuss the role of QMS software in supporting cGMP compliance.
SimplerQMS provides QMS software or eQMS that supports compliance with cGMP regulations. We offer a system designed specifically for Life Science companies. To learn more about our QMS software, book a demo and talk with our quality experts.
The following topics are covered in greater detail in this article:
- What is cGMP?
- What Are the Key cGMP Requirements?
- What Are the Key Areas of cGMP?
- How to Maintain cGMP Compliance?
- What Is the Role of QMS Software in cGMP Compliance?
What is cGMP?
CGMP full form stands for “Current Good Manufacturing Practice.”
CGMP refers to the set of regulations established by the United States Food and Drug Administration (FDA).
The cGMP regulations contain minimum requirements for the methods, facilities, and controls used in the manufacturing, processing, and packing of a product.
CGMP applies to the pharmaceutical, food and beverage, medical devices, and dietary supplement industries. The meaning of cGMP lies in its role in maintaining the safety, efficacy, and quality of products throughout their manufacturing process within these sectors.
What Is the Importance of cGMP?
The importance of cGMP lies in ensuring the consistent quality, safety, and efficacy of products in FDA-regulated industries.
By complying with cGMP regulations, companies can maintain uniform and high-quality manufacturing processes, reduce the risk of product defects or contamination, and meet regulatory and customer requirements.
What Is the Objective of cGMP?
The objective of cGMP is to establish a robust framework that governs every aspect of the manufacturing process, from raw materials to final product distribution, to deliver safe and effective products to consumers.
What Is the Difference Between GMP and cGMP?
The difference between GMP and cGMP is the market they apply to and the level of current practices.
Good Manufacturing Practices (GMP) is a set of requirements applied in various industries, including pharmaceutical manufacturing, to ensure products meet their quality standards.
On the other hand, current Good Manufacturing Practices (cGMP) is a specific set of regulations outlined by the FDA for industries operating within the United States. Accordingly, the “C” in cGMP, which stands for “current,” requires companies to use technologies and systems that are up-to-date to comply with the regulations.
Other differences between GMP and cGMP include quality, cost, and standards, as demonstrated in the illustration below.
Quality
Quality is at the core of both GMP and cGMP requirements, ensuring safe, uniform, and high-quality products.
GMP entails adhering to the required industry standards, while cGMP takes an extra step by keeping up with the latest technologies to ensure updated practices.
Cost
Cost is an inherent consideration in both GMP and cGMP compliance.
However, cGMP tends to be more expensive due to the necessity of additional testing and investments in state-of-the-art technologies to meet the latest standards and requirements.
Current Standard
GMP represents the minimum requirements for product manufacturing, whereas cGMP signifies the advanced and up-to-date regulations that apply to the industry.
Now that we have a clear understanding of what cGMP entails, let’s examine its key requirements to ensure quality and compliance in the manufacturing process.
What Are the Key cGMP Requirements?
The cGMP requirements differ based on the type of product they govern.
Below are the key cGMP requirements applicable to some of the larger Life Science industries.
NOTE
In this section, we will explore some of the cGMP requirements across various industries. However, note that this list is not exhaustive. For official and comprehensive information, always refer to the regulations applicable to your company.
21 CFR Part 111
The 21 CFR Part 111 regulation outlines cGMP requirements specific to the manufacturing, packaging, labeling, and holding operations for dietary supplements.
It covers all aspects of the manufacturing, packaging, labeling, and holding of dietary supplements, including the facilities, equipment, personnel, and procedures used.
The aim is to ensure dietary supplements’ quality, purity, and safety throughout their production and distribution.
21 CFR Part 210
The cGMP requirements for the general manufacturing, processing, packing, and holding of drugs are established in the 21 CFR Part 210 regulation.
It encompasses various aspects of drug production, such as equipment, personnel, and procedures, to ensure consistent product quality and safety.
21 CFR Part 211
The 21 CFR Part 211 specifies cGMP requirements for finished pharmaceutical products.
It covers all stages of pharmaceutical manufacturing, from raw materials to the final packaged products, to guarantee their safety, identity, strength, quality, and purity.
Many Pharma companies operating in the US implement a pharmaceutical quality management system that complies with drug-specific cGMPs, such as 21 CFR Part 211.
21 CFR Part 212
The 21 CFR Part 212 regulation provides cGMP requirements specifically for Positron Emission Tomography (PET) drugs.
It ensures the safe and accurate manufacturing of PET drugs, which are radiopharmaceuticals used in medical imaging.
21 CFR Part 225
cGMP for drugs used in medicated feeds for animal health are outlined in the 21 CFR Part 225 regulation.
It ensures that drugs added to animal food are manufactured, processed, and labeled properly to meet safety and efficacy requirements.
21 CFR Part 606
The 21 CFR Part 606 establishes the cGMP for blood and blood components used in transfusions and medical treatments.
The regulation covers the facilities, equipment, personnel, and procedures used to collect, store, and distribute blood and blood components. It focuses on ensuring the safety and quality of blood products to protect recipients’ health.
21 CFR Part 820
The 21 CFR Part 820, also known as the Quality System Regulation (QSR), sets forth cGMP requirements for medical device manufacturers.
It encompasses various quality management aspects, from design and production to distribution, to ensure safe and effective medical devices. You can learn more about this part of regulation in a dedicated article about 21 CFR Part 820 quality management systems.
For more information on medical device QMS systems, check out our article on Medical Device QMS.
Recognizing the shared key areas of cGMP regulations is essential. However, companies must also grasp the specific requirements pertinent to their operations to develop appropriate processes and controls.
What Are the Key Areas of cGMP?
The areas of cGMP cover various aspects of the manufacturing process to ensure product quality, safety, and efficacy.
Some of the key cGMP areas along with some examples of how SimplerQMS can help manage them are described in more detail below.
Production and Process Controls
This area covers the critical processes involved in manufacturing, including the use of approved procedures, batch records, process validation, and others. Strict controls ensure consistency and reproducibility of product quality.
For instance, to comply with cGMP, companies should maintain detailed batch records with clear documentation of each step taken during production, including critical control points and product sampling.
Written Procedures for Deviations and Nonconformances
Establishing written procedures for handling deviations and nonconformances is essential to address unexpected events or situations that may lead to cGMP noncompliance.
Clear and documented procedures enable companies to investigate deviations and nonconformances thoroughly, determine their root causes, and take appropriate corrective actions.
You can learn more about deviations in the pharmaceutical industry with our dedicated article on deviation management in pharma.
With SimplerQMS, managing deviations or nonconformances (NCs) is an easy task. You can record a deviation or an NC, relate relevant documents to ensure the traceability of information, and directly escalate the issues to Corrective and Preventive Action (CAPA) if needed. The system notifies assigned users of required actions, ensuring timely task completion.
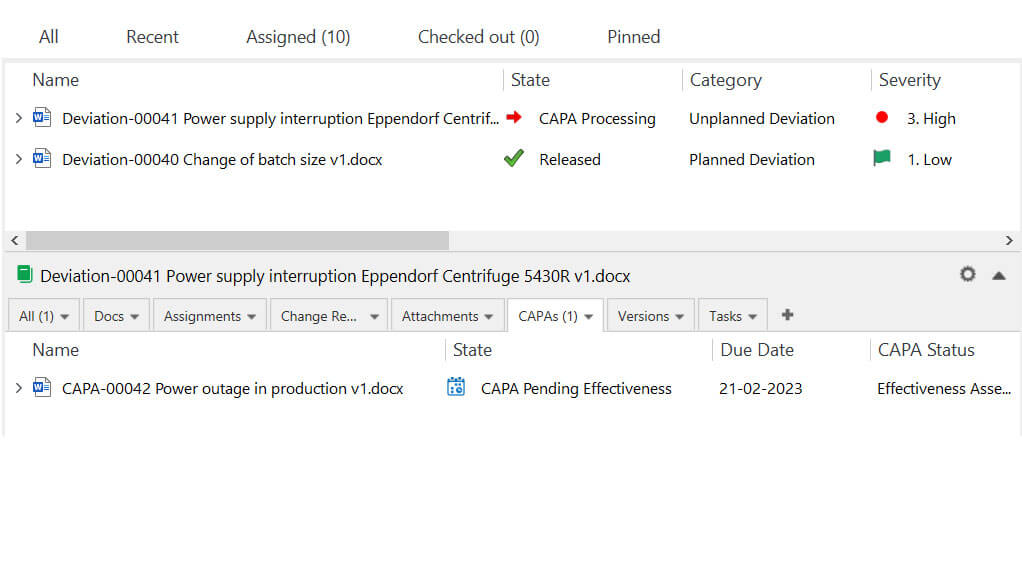
Organization and Personnel
This area focuses on establishing clear roles and responsibilities within the company and ensuring that personnel are appropriately trained and qualified to perform their tasks.
Employees involved in manufacturing, processing, packing, and holding, among many processes, must wear clean and suitable clothing. They should also use protective apparel, for example, head, face, hand, and arm coverings, whenever needed to prevent product contamination.
An example of cGMP for personnel is developing a training plan and conducting regular training sessions to ensure employees understand applicable cGMP requirements, standard operating procedures (SOPs), and safety protocols related to their tasks and responsibilities.
QMS software like SimplerQMS simplifies the creation of training plans, scheduling training assignments, and assigning the appropriate users to training. The system helps ensure timely training completion by sending automated notifications and reminders about the assignment.
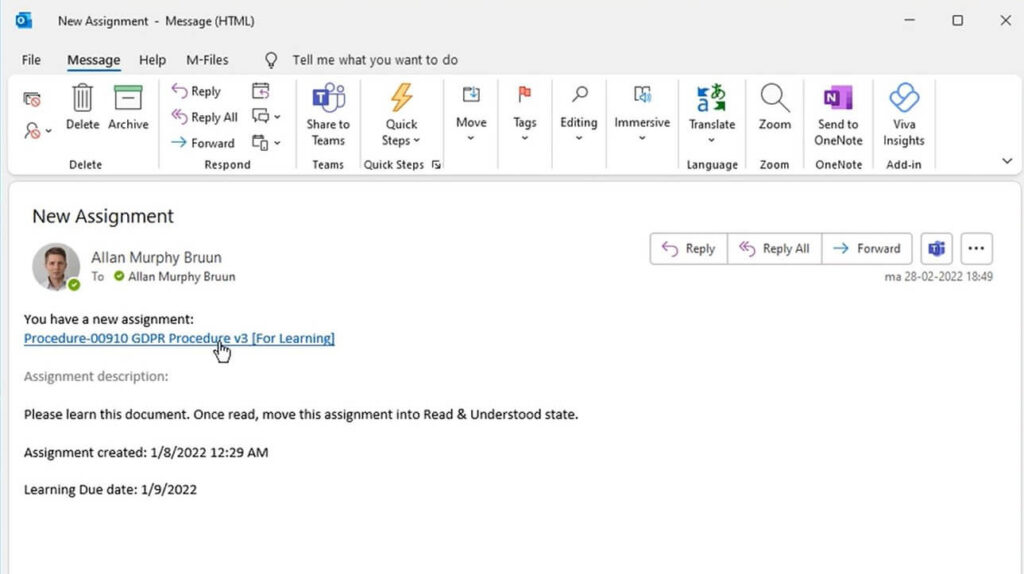
Buildings and Facilities
cGMP regulations require that manufacturing facilities are designed, constructed, and maintained to meet specific cleanliness and safety requirements.
Proper facility design and maintenance help prevent contamination and ensure a hygienic environment for production. This includes cleaning and sanitizing all surfaces regularly, among other procedures.
For example, companies can conduct a risk assessment to identify potential sources of contamination and develop a more comprehensive cleaning and sanitation plan.
Equipment
The regulations emphasize the importance of using well-maintained and calibrated equipment to ensure accurate and reliable results during manufacturing. Proper equipment maintenance reduces the risk of product defects and deviations.
An example of equipment cGMP includes selecting equipment that is made from materials that are compatible with the materials that will be used in the manufacturing process.
SimplerQMS streamlines equipment management by facilitating the creation of calibration and maintenance plans. Our solution sends automatic notifications and reminders to responsible people to perform their assignments on time, helping ensure that equipment is always in good working order.
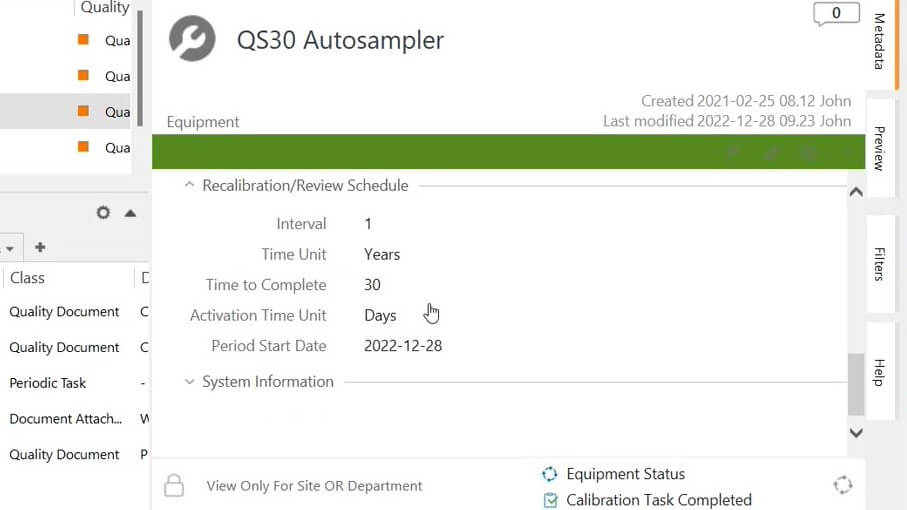
Records and Reports
Accurate and comprehensive recordkeeping is essential in cGMP compliance. Companies are required to maintain detailed records with evidence of all manufacturing and quality control activities to demonstrate compliance with regulations.
An example of cGMP could be a well-implemented and robust document management system that controls versions of SOPs, batch records, and other cGMP-process-related documents.
Implementing a QMS that reflects cGMP requirements in the key areas is essential for maintaining compliance throughout the manufacturing process.
With SimplerQMS, it is easy to keep track of document versions. The software automatically creates new versions of documents, along with a time-stamped audit trail for every change that shows the document version, and the responsible person for the change, date, and time.
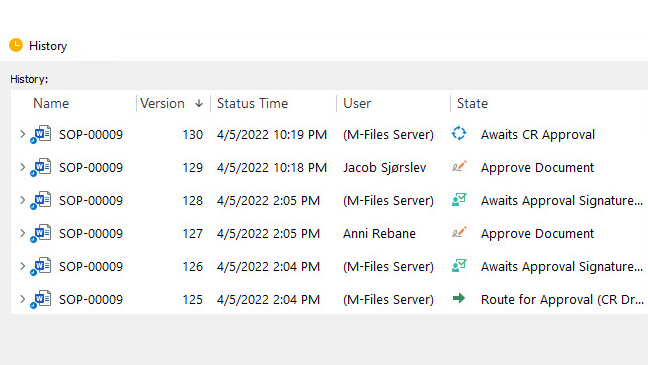
How to Maintain cGMP Compliance?
Maintaining cGMP compliance requires management commitment and following activities as specified in the applicable requirements.
By following the key actions below, companies can ensure continuous compliance with cGMP requirements.
- Internal audits: Companies should conduct regular internal audits to assess compliance with cGMP requirements and identify potential areas of noncompliance. Internal audits should be conducted by a competent team to review processes, procedures, and systems. The audit findings should be documented, and deviations must be addressed with corrective and preventive actions (CAPAs) to prevent recurrence, if necessary.
- Ongoing training: Continuous training must be provided for employees to ensure they are well-informed about cGMP regulations, SOPs, and best practices. Properly trained staff are more likely to follow procedures accurately, reducing the risk of errors. Regular training sessions keep employees updated on any changes to regulations.
- Maintaining effective documentation: Critical processes, data, and quality-related activities must be documented appropriately and retained securely. Proper documentation includes batch records, SOPs, calibration records, validation reports, and other cGMP-related documents. Well-maintained records provide evidence of compliance during inspections and help identify deviations or trends for continuous improvement.
- Maintaining equipment in good condition: Equipment used in manufacturing, testing, and quality control processes must be well-maintained and calibrated to ensure accurate and reliable results. Implementing preventive maintenance plans prevents unexpected equipment failures, helps extend the lifespan of the equipment, and minimizes the risk of cGMP deviations due to equipment-related issues.
- Perform periodic reviews: Companies should schedule routine reviews to evaluate the adequate functioning of processes and procedures in compliance with cGMP requirements. Reviews include a comprehensive assessment of process execution. Findings from these reviews must be documented, enabling informed decision-making and continuous improvement.
These actions help maintain compliance with cGMP by organizing a system that effectively manages cGMP-related processes. Additionally, implementing QMS software can streamline quality processes and further support cGMP compliance.
What Is the Role of QMS Software in cGMP Compliance?
QMS software supports Life Science companies in achieving and maintaining cGMP compliance by automating tasks, facilitating employee training, and helping identify areas of improvement, among many other things.
SimplerQMS provides an eQMS software solution designed to support cGMP compliance.
Although cGMP processes can be managed manually using paper-based or hybrid systems, these can lack document and record control, be time-consuming, and error-prone.
An improved approach involves the implementation of eQMS software with pre-defined workflows tailored to life science industries, guiding users through the intricacies of diverse QMS processes and facilitating adherence to regulatory requirements.
Here are some examples of key process areas, along with how SimplerQMS software helps ensure cGMP compliance for each one:
- Document Control: Our eQMS provides pre-defined workflows that guide users through actions like document reviews and approvals with electronic signatures, ensuring appropriate action is taken. It also tracks all document changes and versions, along with time-stamped audit trails.
- Change Control: SimplerQMS helps Life Science companies create, document, and manage all changes. We provide a centralized platform for managing change requests, tracking the status of changes, and ensuring that all changes are reviewed and approved before they are implemented.
- Training Management: Our software simplifies employee training while supporting compliance with Life Science requirements. SimplerQMS streamlines learning processes by automating assignments, reminders, and status tracking, as well as helping ensure that everyone is trained on the latest procedures.
- Equipment Management: SimplerQMS simplifies equipment registration, calibration, and maintenance tasks. Our system allows you to categorize and monitor equipment and set up task schedules with automated notifications and reminders.
Documentation in SimplerQMS is securely signed using 21 CFR Part 11-compliant electronic signatures to promote accountability within these and more processes.
Apart from the processes mentioned above, the SimplerQMS solution supports other QMS processes that include CAPA management, customer complaint management, audit management, supplier management, and more.
By providing such QMS process support, the SimplerQMS platform supports compliance with cGMP and several other Life Science requirements, which include ISO 9001:2015, ISO 13485:2016, FDA 21 CFR Part 11, EU GMP Annex 11, EU GMP, and more. By providing QMS process support, SimplerQMS software helps companies comply with regulatory requirements.
If you are considering implementing an eQMS solution in your company, we have a free eQMS Business Case template for you to download.
The template will assist you in calculating the economic value and time saving that an eQMS can bring, enabling you to effectively present your findings to management.
Final Thoughts
CGMP, or “Current Good Manufacturing Practices,” are regulations established by the FDA to ensure consistent production and control of products in industries like pharmaceuticals, food and beverage, medical devices, and dietary supplements.
Some companies use paper-based or hybrid systems to manage cGMP processes. However, these can be time-consuming and prone to errors.
A better solution is using eQMS software, like SimplerQMS, which provides comprehensive QMS process support. Our pre-defined workflows guide users through processes to ensure the completion of tasks in an orderly manner facilitating adherence to regulatory requirements.
SimplerQMS provides eQMS software specifically designed to support cGMP compliance and several other Life Science requirements, such as ISO 9001:2015, FDA 21 CFR Part 11, FDA 21 CFR Part 211, FDA 21 CFR Part 212, EU GMP Volume 4 Annex 11, EU GMP Volume 4 Part I, and more.
If you are interested in learning more about our eQMS software, you can book a free demo and talk to our Quality Solution consultants.