QMS documentation discussed in this article are documents and records related to compliance and quality assurance. These documents define the company’s Quality Management System (QMS).
The QMS documentation is defined by each company based on regulatory and customer requirements.
This article will discuss QMS documentation, its structure, requirements, benefits of well-documented and implemented documentation, and maintenance. It also explains the role of QMS software in managing QMS documentation effectively.
Life Science companies are increasingly adopting electronic Quality Management Systems (eQMS) to streamline their quality documentation processes.
SimplerQMS provides eQMS software with comprehensive document management capabilities designed for Life Science companies. Schedule a free demo and talk to our quality experts to get a more comprehensive understanding of the QMS software.
We will explore the following topics in more detail.
- What is Quality Management System (QMS) Documentation?
- What is QMS Documentation Structure?
- What Are the QMS Documentation Requirements?
- What Are the Best Practices for QMS Documentation?
- How to Ensure Effective QMS Documentation?
- How to Ensure Continuous Improvement in the QMS Documentation?
- What Are the Essentials for Effective Quality Management System Documentation?
- What Are the Benefits of Well-Documented and Implemented QMS Documentation?
- What Is the Role of QMS Software in Managing Quality Documentation?
What is Quality Management System (QMS) Documentation?
The QMS documentation comprises a set of documents and records that define the company’s Quality Management System (QMS). QMS documentation includes documents related to compliance with applicable requirements, as well as to quality assurance.
The QMS documentation provides a structured approach to quality management, regulatory compliance, and continuous improvement.
The main goal of a QMS is to help ensure that the company complies with applicable requirements. The QMS documentation includes records of how activities were performed as evidence of compliance.
The QMS documentation can be paper-based, hybrid, or electronic. Paper-based and hybrid QMS documentation presents some challenges, such as lack of document control, lack of control of records, lost documents, the need for physical storage, and increased potential for human error.
Software solutions, like SimplerQMS, offer a modern way of working with QMS documentation. Life Science companies can manage and streamline QMS documentation using automated workflows, cloud storage, electronic signatures, and more.
The company must decide which documents are included in the QMS documentation based on relevant requirements. Some examples of QMS documentation include documents that describe a company’s quality policies, processes, procedures, and work instructions.
What is QMS Documentation Structure?
QMS documentation structure is a hierarchical organization of documents within the QMS. The documentation hierarchy makes it easy to understand, communicate, and visualize the documentation structure.
Each documentation level in the structure builds upon the previous one and contributes to the overall effectiveness of the QMS.
As an example, the four levels of documents in the QMS pyramid could include the following:
- Quality policy
- Procedures
- Work instructions
- Records
Quality Policy
The quality policy is a statement that defines the company’s commitment to quality. It is a high-level document outlining the values and principles regarding quality and providing a framework for setting quality objectives.
At SimplerQMS, we advocate for creating quality policies that clearly state the company’s desire to support high and uniform quality in products and processes. It is important for people reading the Quality Policy to see your company’s identity reflected in your quality policy.
Procedures
A procedure describes the step-by-step activities of processes within the company. It includes elements such as the responsible departments or functions and the frequency of the action.
These procedures provide clear guidelines, helping achieve efficiency, quality output, and consistent performance while reducing miscommunication and noncompliance with relevant requirements.
Work Instructions
Work instructions are the most detailed documents in the QMS structure. They describe in detail how specific tasks must be performed.
They are typically written by the people who perform the work or people who are responsible for leading those who perform the work. Work instruction can be developed in the company or provided by customers.
The instructions help ensure tasks are carried out consistently and effectively and meet the applicable requirements of the quality management system.
Records
Records provide evidence that activities and events were conducted, providing a historical record of actions.
By performing internal audits and reviewing the records, companies can support evidence-based decision-making and demonstrate compliance with requirements.
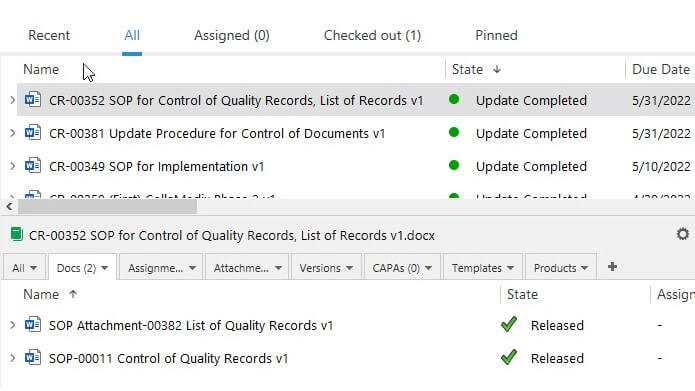
What Is the Difference Between Records and Other Quality Documents in QMS?
The difference between records and other types of documents is that records are evidence of activities performed or results achieved, while other quality documents mostly describe how tasks should be done.
For example, a work instruction describes how a process must be performed, while a record could show how the process was performed, who did it, when, and the result.
Another distinction between records and other types of documents is that records remain unaltered. In contrast, other documents are subject to changes and revisions over time.
As records remain unchanging, it is important to reference the specific versions of the quality documents that were in effect when the record was generated. This ensures that the records accurately reflect the correct QMS documentation at a given time.
SimplerQMS provides eQMS software with comprehensive document control capabilities. New document versions are automatically numbered when created or updated. The system makes it easy to set relations between documents or add links using hyperlinks.
What Are the QMS Documentation Requirements?
The QMS documentation requirements vary depending on the industry. Requirements define which documents must be included in the QMS.
The extent of QMS documentation for a quality management system can differ from one company to another due to its type of activities, processes, products, and services.
The International Organization for Standardization (ISO) provides general guidance for developing and maintaining documentation in the ISO 10013:2021.
The standard gives guidance for managing QMS documentation necessary to support an effective quality system tailored to the company’s specific needs, regardless of the industry in which it operates.
Companies operating in the Life Sciences industries, such as pharmaceutical and medical device companies, must comply with applicable requirements.
NOTE
We will explore some of the QMS documentation requirements in this section. However, note that this list is not exhaustive. For official and comprehensive information, always refer to the requirements applicable to your company.
What Are the Key QMS Requirements for Pharmaceutical Companies?
ISO 9001:2015
The international standard ISO 9001:2015 specifies the requirements for quality management systems. The ISO 9001:2015 standard requires companies to maintain and retain documented information to support their processes and ensure they are executed as planned.
ISO 9001:2015 is a general standard for quality management systems, and many pharmaceutical companies follow this standard as it provides a structured framework for QMS.
In addition to the requirements of ISO 9001:2015, companies may also have to comply with specific requirements from GxP and/or other requirements.
Examples of QMS documentation required by ISO 9001:2015 include quality policies, quality objectives, design and development planning, nonconformance documents, customer complaints, and more.
FDA 21 CFR Part 211
The FDA regulation 21 CFR Part 211 is the current Good Manufacturing Practice (cGMP) for finished pharmaceuticals.
Regarding QMS documentation, it specifies the requirements for written procedures, records, and reports to be managed and kept readily available for inspection during the retention period.
This regulation requires QMS documentation, such as batch production records, equipment use logs, complaint files, and sanitation procedures, among others.
Read our introductory guide to cGMP if you are interested in learning more about cGMP regulations.
ICH Q10
The ICH Q10 is a guideline describing a model for an effective quality management system for the pharmaceutical industry.
It outlines the requirements for the quality manual, CAPA documents, product discontinuation reports, and more. Compliance with ICH Q10 ensures that the documentation of the pharmaceutical quality management system is well-structured and clear, promoting common understanding and consistent application.
Examples of QMS documents in the ICH Q10 include quality manual, quality policy, CAPA documents, product discontinuation documents, deviation documentation, and more.
Have a look at our comprehensive ICH Q10 article to gain a better knowledge of the pharmaceutical quality system.
What Are the Key QMS Requirements for Medical Device Companies?
ISO 13485:2016
The regulatory standard ISO 13485:2016 outlines the requirements for medical device quality management systems.
Concerning QMS documentation, ISO 13485:2016 requires companies to create and manage documents and records to ensure the effective planning, operation, and control of quality processes.
This standard requires QMS documentation to include quality manuals, medical device files, training records, and complaint handling records, among others.
You can learn more about the ISO 13485:2016 quality system by reading our article about ISO 13485:2016 compliant QMS.
FDA 21 CFR Part 820
The 21 CFR Part 820, also known as the Quality System Regulation (QSR), establishes the requirements applicable to manufacturers of finished medical devices.
The regulation requires manufacturers to establish and maintain procedures to control all documents related to medical devices, including the creation, review, approval, and change processes.
For instance, 21 CFR Part 820 requires QMS documents such as design history files, device master records, nonconformance documents, equipment calibration records, CAPA documents, and more.
Check out our article on medical device quality management systems to learn more about the key requirements and major processes.
What Are the Best Practices for QMS Documentation?
Implementing general best practices for QMS documentation development helps ensure it is accurate, up-to-date, accessible, and easy to understand.
Here are some examples of general best practices when developing QMS documentation.
Make Sure Your Documentation Is Fit-For-Purpose
The QMS documentation should be tailored to the specific needs of the company and its processes, as well as to the intended user’s needs.
Use Clear and Concise Language
The language in the QMS documentation should be clear, concise, and easily understandable.
Avoid technical jargon or complex terminology that may impact comprehension. Aim for simplicity to improve clarity and promote effective communication.
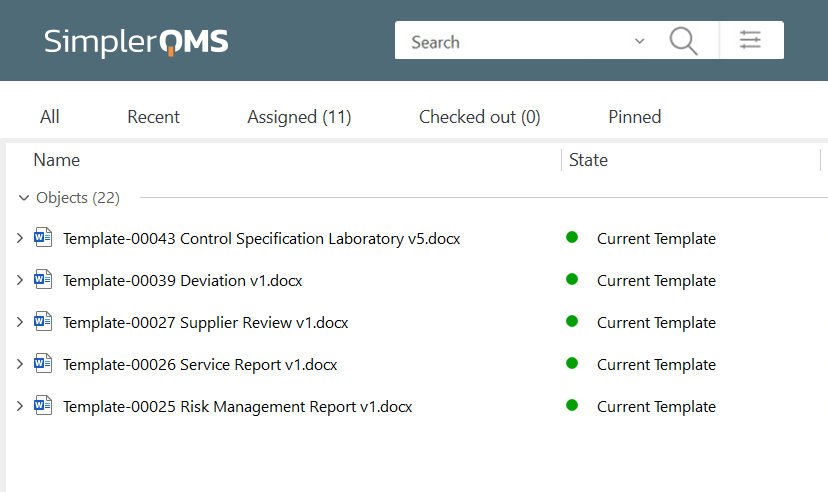
Implement document templates to ensure consistency in process descriptions and instructions across all documents.
For example, SimplerQMS offers eQMS software with the ability to use highly customizable documentation templates for document creation. Companies can use our complementary template package or their existing templates.
Use a Distinct Structure
Documentation must have a clear and logical structure that makes information easy to find. The structure must be consistent throughout the QMS documentation so that users can easily navigate between different documents.
Keep Your Documentation Lean
QMS documents need to be as concise as possible without sacrificing clarity or completeness. Companies should prioritize including only necessary information in the documentation and avoid unnecessary details, redundancies, and lengthy explanations.
Use Visuals
Visuals can be included to help make QMS documentation easier to understand. Companies can incorporate visual aids such as flowcharts, diagrams, and tables to convey complex information efficiently.
Review and Update Your Documentation Regularly
QMS documentation must be regularly reviewed and updated to reflect the company’s current processes, procedures, or requirements changes.
How to Ensure Effective QMS Documentation?
The effectiveness of QMS documentation can be ensured through the following processes:
- Internal audits
- External audits
- Management reviews
Below is a more detailed explanation of how these processes can help to ensure effective QMS documentation.
Internal Audit
Internal audits are conducted by the company itself to identify any gaps or inconsistencies in its QMS documentation and to verify that employees are working in accordance with procedures.
This involves evaluating processes, procedures, and work instructions to verify compliance with applicable requirements. Relevant people conducting the audit may also check records and monitor whether employees work according to procedures in person.
Analyzing records is important to collect evidence that employees are following quality documents and to identify areas for improvement within processes.
External Audits
External audits are conducted by independent auditors, such as certification bodies. These audits assess the adequacy and effectiveness of QMS documentation and alignment with applicable regulatory requirements.
For example, an external audit could be conducted to assess the company’s QMS documentation and its compliance with ISO 9001:2015.
Management Review
Management reviews are conducted by top management to monitor and define the effectiveness, suitability, and adequacy of QMS documentation. Management reviews help to ensure that the documentation is aligned with the company’s quality goals and regulatory requirements.
Not all companies are required to implement management reviews depending on their applicable requirements. Some examples of requirements that specify management review are ISO 13485:2016, 21 CFR Part 820, and ICH Q10.
For companies to whom management reviews apply, management reviews should be periodically performed to assess the overall performance of QMS documentation, typically once a year. This involves evaluating the QMS for effectiveness and adequacy and ensuring sufficient resources for the intended use.
While performing audits and reviews, Life Science companies can detect and address issues within their QMS, allowing them to implement essential improvements as needed.
How to Ensure Continuous Improvement in the QMS Documentation?
To ensure continuous improvement in the QMS documentation, Life Science companies can utilize the PDCA (Plan-Do-Check-Act) cycle.
The PDCA management method consists of four steps:
- Plan your actions
- Do what you planned
- Check that everything has been done correctly
- Act to implement necessary improvements
The cycle can be repeated as often as necessary to achieve the desired results. It is a powerful tool for continuous improvement as it facilitates learning from mistakes and incorporating corrective actions based on acquired knowledge.
The process of structuring and maintaining QMS documentation within an effective quality management system follows the PDCA cycle.
Following the PDCA cycle, illustrated below, Life Science companies can establish a systematic approach to ensure continuous improvement in the QMS documentation.
What Are the Essentials for Effective Quality Management System Documentation?
Effective QMS documentation requires several essential components to ensure accuracy, accessibility, compliance, and operational efficiency.
The following are some essential processes for creating and maintaining effective QMS documentation, along with examples of how an eQMS such as SimplerQMS further streamlines these processes.
Document Control
Document control is a requirement for all companies and must be in place to manage document creation, review, approval, distribution, and archiving.
This helps ensure that the documentation is controlled, consistently maintained, and organized.
Many regulatory requirements compel companies to have a document management process.
SimplerQMS provides comprehensive document control capabilities, providing pre-defined workflows for document creation, review, and approval. These workflows serve as a guiding framework, assisting users throughout the documentation processes and helping ensure adherence to Life Science requirements.
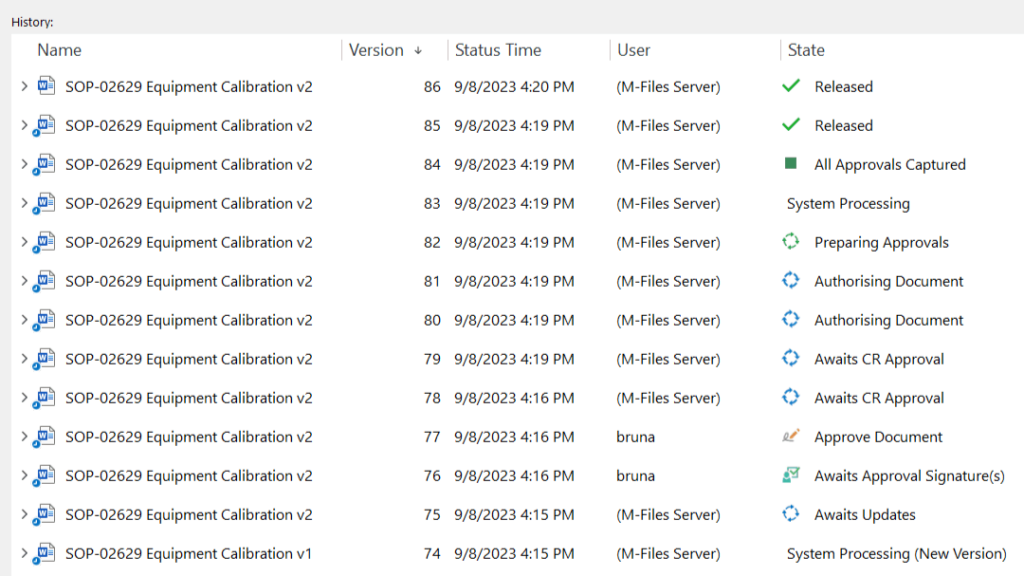
Implement Version Control
Version control must be implemented to track and manage document revisions.
Clearly indicate the document version number, who modified the document, and the date and time to avoid confusion and ensure the latest version is readily identifiable and accessible.
Versioning of documents is automatically done in SimplerQMS. New or updated documents receive a sequential number for the identification of the latest version. The old document is retired to avoid the use of outdated documents.
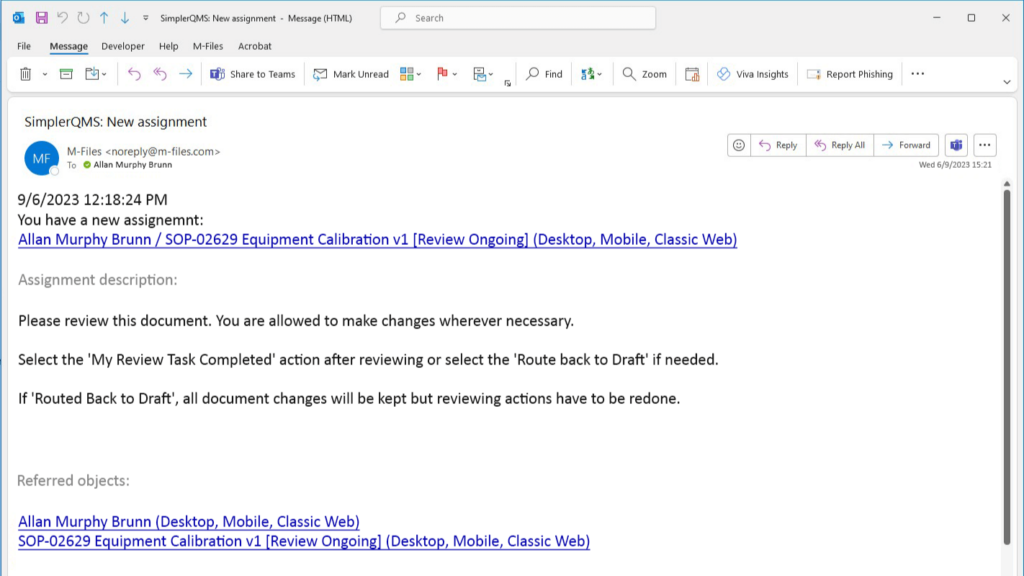
Streamline Document Review and Approval
A formal document review and approval process should be utilized to ensure accuracy and compliance with regulatory requirements.
Involve subject matter experts in the review process to obtain valuable input.
With SimplerQMS, document review and approval processes become effortless. The pre-configured system workflows route the documents to assigned personnel together with notifications of required actions.
Ensure Document Accessibility
Only authorized personnel should have access to the required documentation.
Implement a document management system to facilitate document access, search, retrieval, and distribution.
SimplerQMS ensures that access to the system is granted only to authorized individuals, preventing unauthorized access. Employees have unique identification codes and password combinations that periodically need to be updated.
Employ Document Change Management
The change management process needs to be well-defined for document modifications and updates.
For companies that require comprehensive document change management, this process could include steps for reviewing proposed changes, obtaining approvals, communicating changes to relevant persons, and ensuring adequate implementation.
A more rigorous document change management process is necessary for quality documents, as it ensures changes in critical procedures and instructions are made in a controlled and systematic manner.
The SimplerQMS software offers robust change management capabilities. It facilitates the generation, documentation, and management of all changes across the company. Tasks can be created and assigned, electronically signed upon approval, and automated notifications and reminders can be established to ensure the timely fulfillment of change-related processes.
Provide Employee Training
Comprehensive training plans must be conducted to educate employees on the purpose, content, and use of documentation. A skill matrix should be in place to assess employees’ competencies, identify skill gaps, and support the creation of training plans.
By providing effective training, companies equip employees with the necessary skills and knowledge to create, manage, and utilize documentation effectively throughout their roles.
For instance, the SimplerQMS Employee Training Management module allows companies to achieve efficient, well-documented, and compliant training processes. This module includes features such as automated assigning of training activities, email notifications and reminders, training effectiveness assessments, tracking of training progress, and much more.
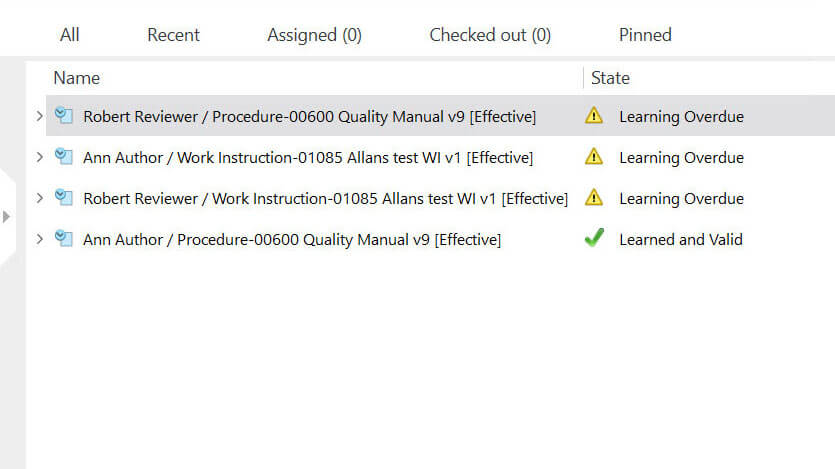
Conduct Internal Audits
Regularly perform internal audits of the QMS documentation to verify compliance with established procedures and standards. As mentioned above, these audits help identify any issues, gaps, or areas for improvement within the QMS documentation.
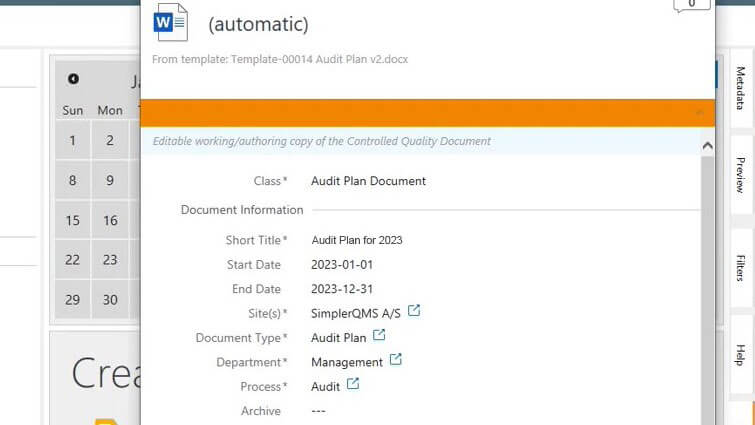
With the SimplerQMS Audit Management module, you can create comprehensive audit plans. Create regulatory inspections, supplier audits, and internal and external audits, link them to audit plans, and relate any relevant documents. Moreover, you can monitor all activities associated with audits, ensuring a comprehensive approach to audit management.
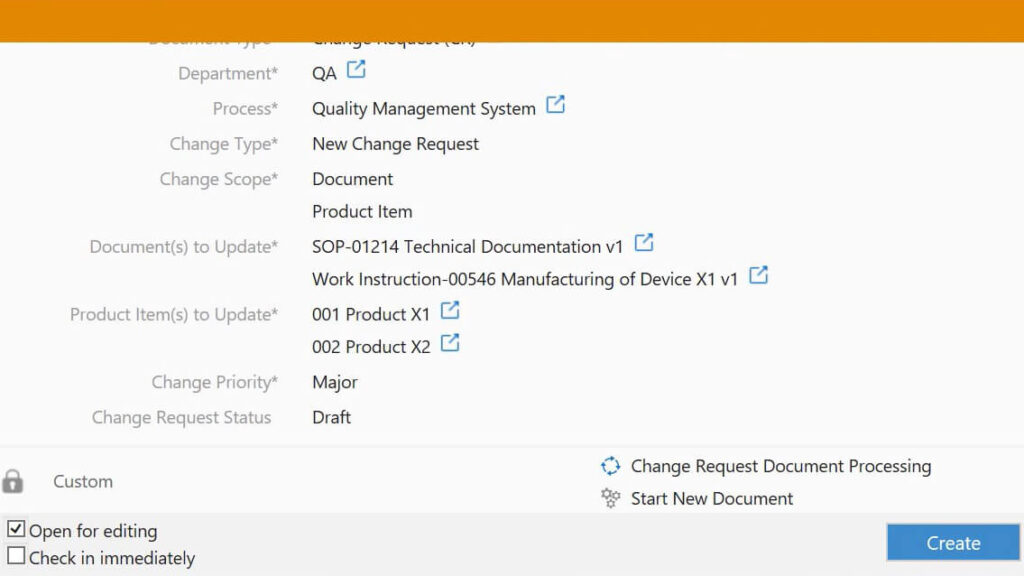
What Are the Benefits of Well-Documented and Implemented QMS Documentation?
Some of the key benefits of having adequate QMS documentation are listed below.
- Compliance with requirements: When QMS documentation content follows the applicable requirements it helps companies demonstrate compliance.
- Improved efficiency: Adequate QMS documentation helps to streamline processes and reduce errors, which leads to increased efficiency.
- Reduced costs: Effective documentation facilitates identifying and mitigating risks, reducing the risk of recalls and other regulatory issues.
- Improved communication: Accurate and clear documents ensure everyone understands the quality processes and quality procedures, improving communication and collaboration.
- Increased customer satisfaction: QMS documentation helps to ensure that products and services are uniform and high quality to meet customer requirements.
- Improved decision-making: Effective QMS documentation can provide comprehensive information needed to make informed decisions about quality processes.
While the benefits of QMS documentation are evident, creating and maintaining effective documentation manually presents challenges. QMS software helps to streamline the process and ensure that documentation is accurate, up-to-date, and accessible.
What Is the Role of QMS Software in Managing Quality Documentation?
QMS software plays an essential role in managing quality documentation.
Even though traditional paper-based and hybrid systems can be used by small companies with sufficient resources, these present challenges and limitations. For example, lack of document and record control, lost documents, and the need for physical storage space can lead to inefficiencies and potential data loss.
Due to the complexity involved in the documentation processes, traditional quality systems are being replaced by electronic solutions. Utilizing the eQMS streamlines managing documentation while reducing the risk of lost documents and compliance issues.
SimplerQMS is a fully validated eQMS software solution for Life Sciences designed to simplify and optimize the management of quality documentation, offering a range of features and benefits.
We provide Quality Document Management Software that streamlines the management of QMS documentation. Our system automates workflows for document creation, review, and approval. You can set automatic reminders and notifications for document updates and ensure quality documents comply with applicable regulations and standards.
An essential aspect of QMS documentation is the approval of documents through electronic signatures. SimplerQMS provides 21 CFR Part 11 compliant electronic signatures, allowing secure signing of documents. This process involves unique combinations of user identification codes and passwords to ensure the authenticity and equivalence of electronic signatures to handwritten ones.
Besides 21 CFR Part 11, our QMS platform supports compliance with several Life Science requirements, including ISO 9001:2015, ISO 13485:2016, FDA 21 CFR Part 210, 211, and 820, EU GMP Annex 11, EU GMP, and more. By providing comprehensive QMS process support, SimplerQMS software helps Life Science companies comply with various regulatory requirements.
Some of the QMS processes supported by the SimplerQMS solution include document management, change control, employee training management, CAPA management, customer complaint management, audit management, supplier management, and more.
We suggest downloading our eQMS Business Case template to evaluate the benefits of implementing an eQMS.
You can use this tool to assess the value of an eQMS for your company. Furthermore, it allows you to effectively communicate your findings to management. Such analysis can help you identify potential efficiency increases, cost reductions, and compliance improvements.
Final Thoughts
QMS documentation comprises the documents and records that establish the company’s Quality Management System (QMS). QMS documentation is related to compliance with regulatory and customer requirements and quality assurance aspects.
Electronic systems are replacing paper-based and hybrid systems for managing quality documentation as they offer several benefits. QMS software presents features that streamline QMS documentation management, improving effectiveness and compliance efforts.
SimplerQMS presents QMS software with robust quality management capabilities tailored for Life Science companies. Interconnected processes and pre-defined workflows guide users through the core stages of each process, providing support in ensuring compliance with Life Science requirements.
Learn more about how the SimplerQMS solution can support your company. Book a free demo and talk with our quality experts to better understand how our QMS software can help you streamline your QMS documentation management.