Risk Management Solution for Life Sciences
Consolidate and organize risk management documentation throughout all quality processes.
TRUSTED BY
























Risk Management Within a Comprehensive eQMS
The SimplerQMS Risk Management module allows Life Science companies to control and manage risk management documentation. The system promotes document traceability and consistency in risk assessment procedures across all quality processes.
By linking related documents like SOPs, training records, and risk assessments, you can gain a clear picture of how risks are identified, mitigated, and controlled.
The risk management module is part of an all-in-one eQMS solution designed for the needs of the Life Science industry. SimplerQMS software encompasses all QMS modules, including document control, change control, training, supplier management, audit management, CAPA management, and more.
Handle Risk Management Documentation
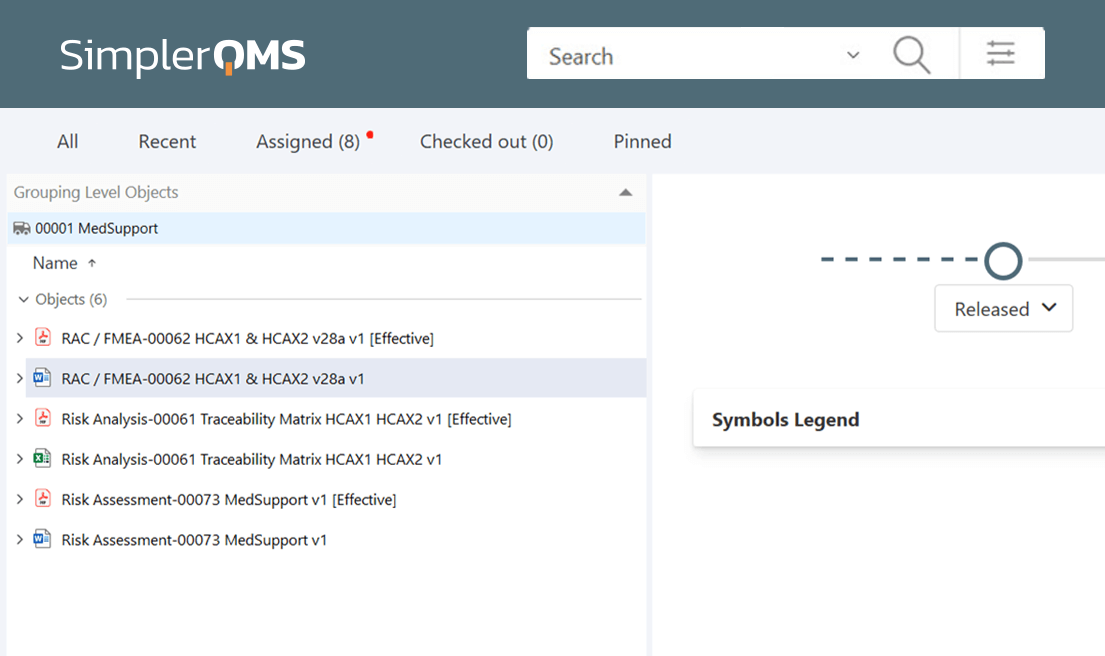
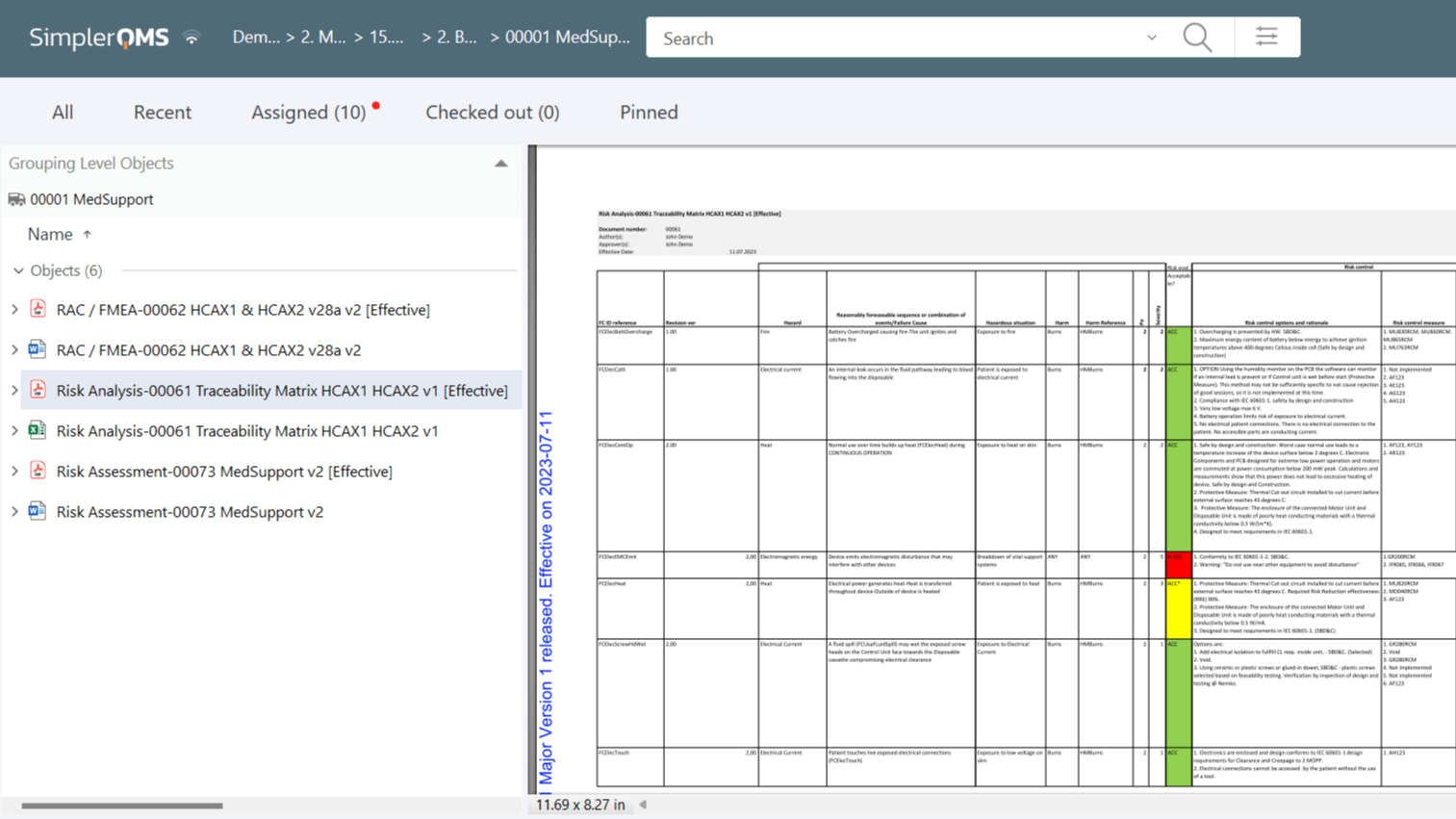
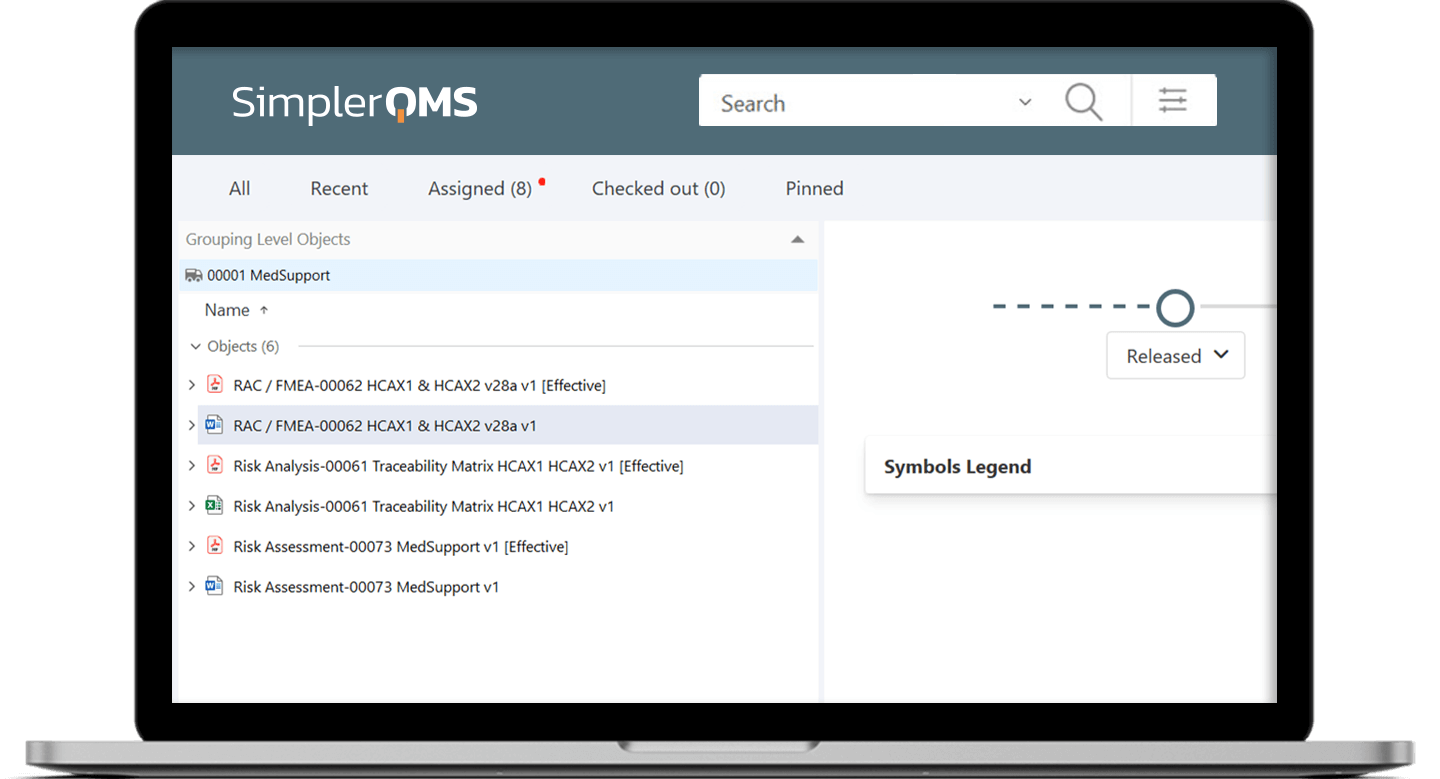
SimplerQMS offers risk management integrated with the document management system. Streamline the management of Risk Analysis, Risk Assessments, and Risk Assessment and Control (RAC) documents in one single solution.
Utilize automated workflows that guide users in handling risk management documentation while supporting compliance with relevant requirements. Maintain full visibility into the entire workflow and have access to information regarding the next steps at all times.
Utilize Risk Document Templates
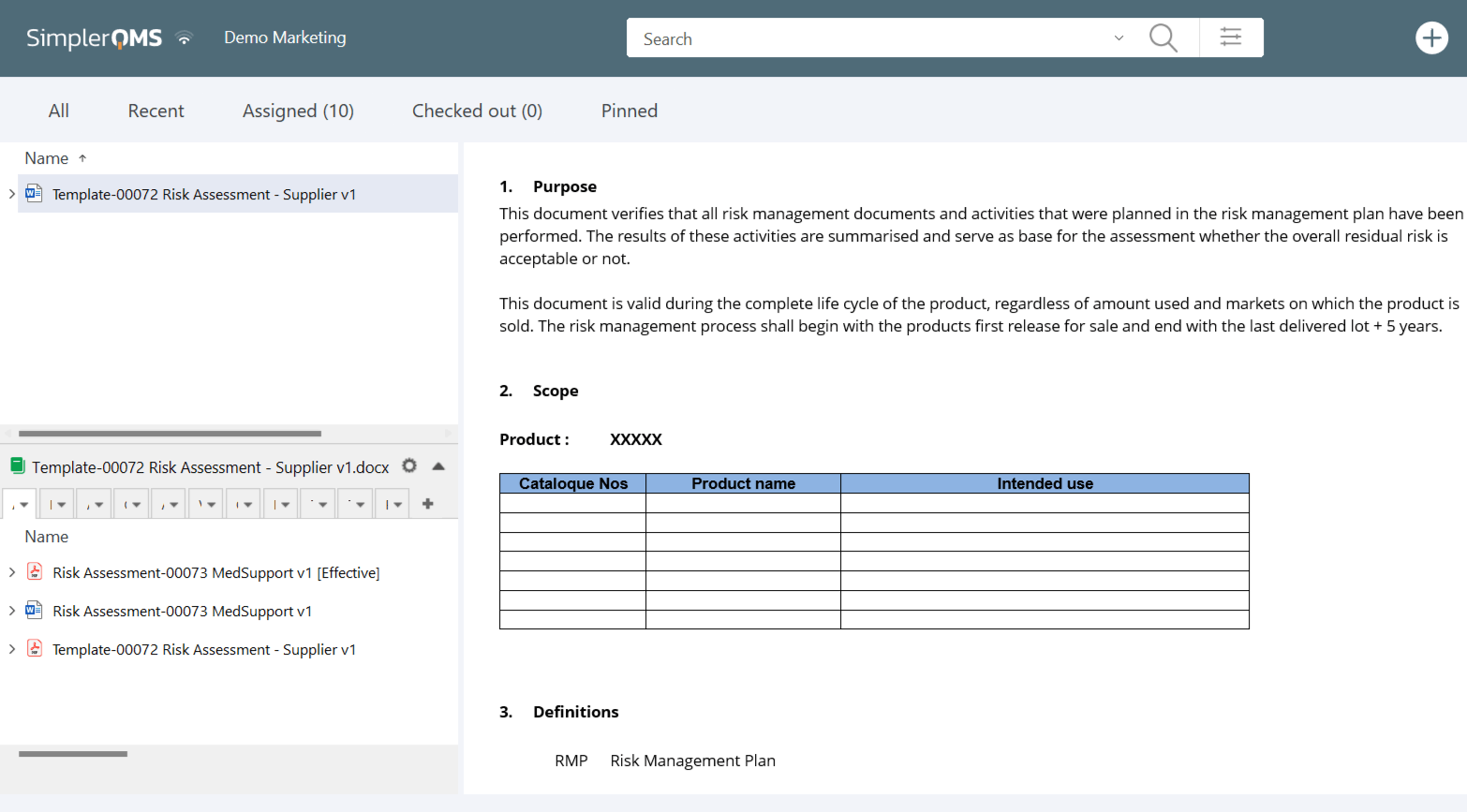
Create risk management documents, from risk assessments and risk management plans to traceability matrixes, using the SimplerQMS complimentary template package.
Utilize pre-designed forms and templates, or tailor them to match your unique needs.
You can also migrate your existing templates with the drag-and-drop functionality. Drag and drop Word, Excel, or PDF document templates directly into the system.
Relate Risk Management Information
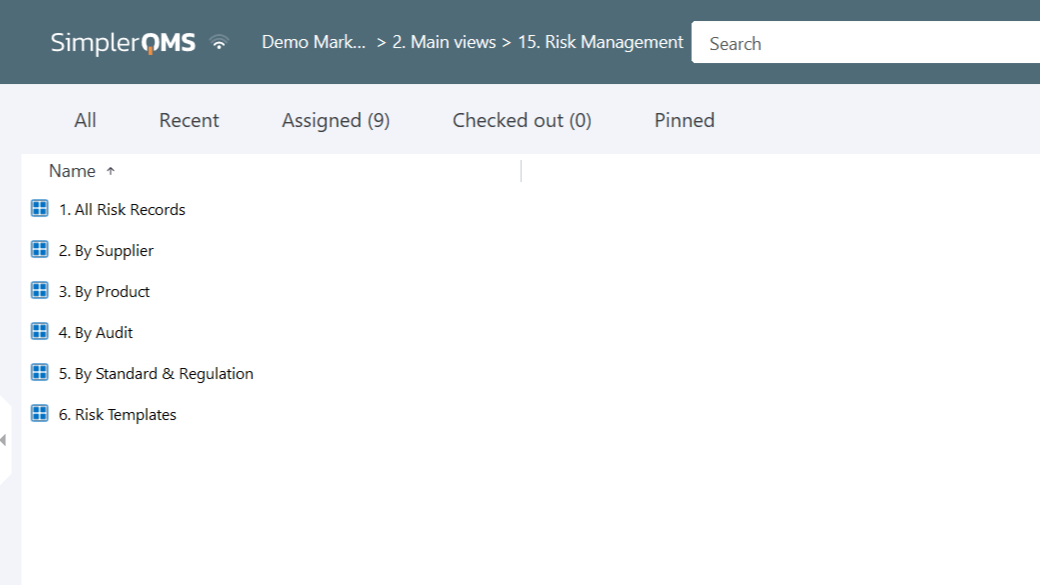
Relate risk management data to all quality processes. Easily create links between risk management files and other documents, records, and items within the system. Ensure data traceability and easy access to risk documentation.
Personalize risk management views within all quality processes to consolidate and organize risk data related to suppliers, products, audits, projects, nonconformances and deviations, and more.
Evaluate Risk Periodically
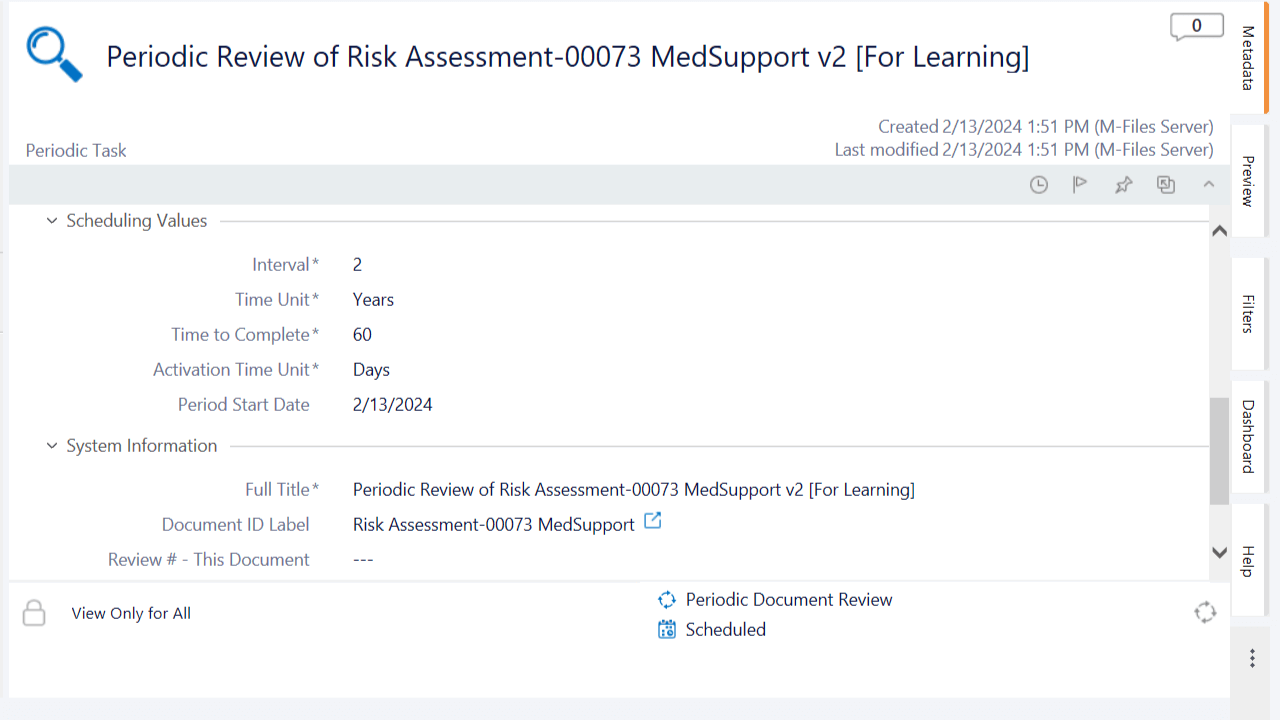
Create risk management plans to analyze, evaluate, and monitor risk when the need arises. Link risk assessments and risk analysis to nonconformances and deviations to reevaluate risk after issues have been recorded.
Schedule periodic actions to review risk assessments. Ensure timely completion of these actions with automatic notifications and reminders to assigned personnel.
Manage Risk With Traceability Matrix
SimplerQMS provides a traceability matrix document template to help control risk assessments and mitigation processes throughout the lifecycle of a product or process.
Utilize relations and hyperlinks within the traceability matrix to connect several elements in the system, such as risk assessments, risk mitigation strategies, nonconformances and deviations, CAPAs, and more.
Link specific versions of a document or the latest version of a document in the traceability matrix to create a record of all risk-related actions.
Present Risk Documents During Audits
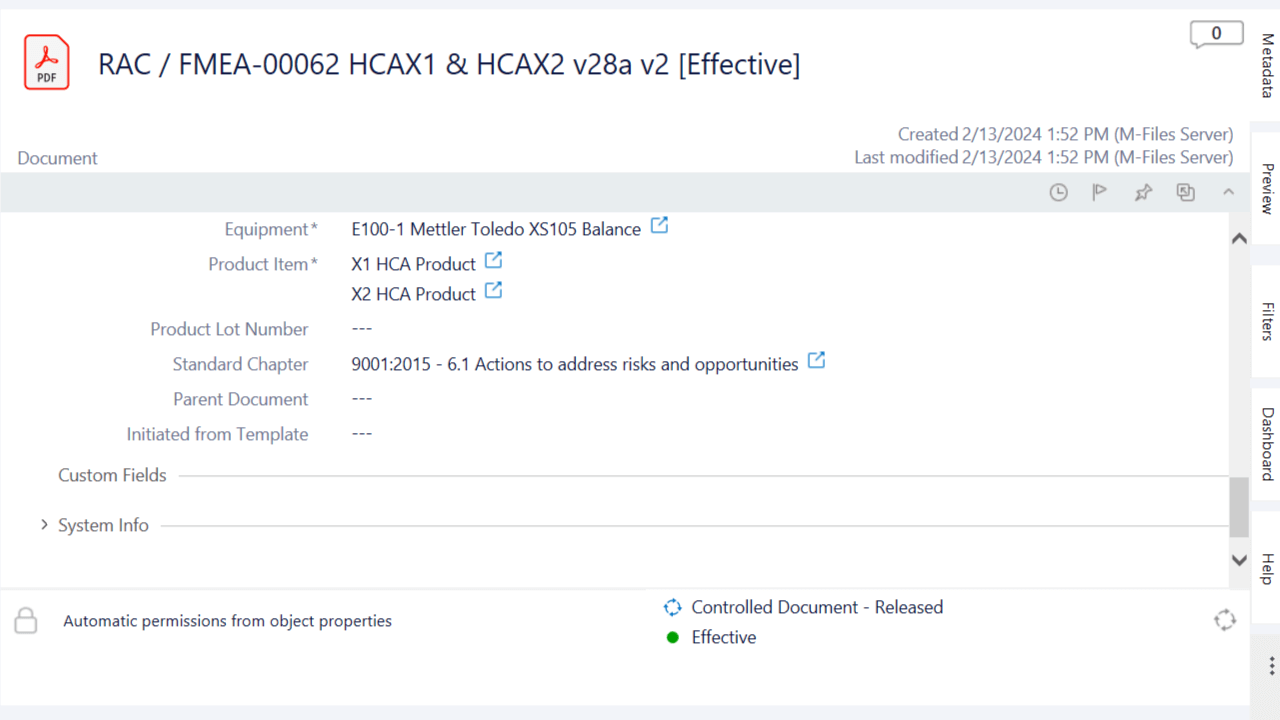
Facilitate the retrieval and presentation of risk management documentation during audits and inspections by relating risk documents to regulatory requirements, such as standard chapters.
Link risk management documents to relevant regulatory requirements in just a few clicks when drafting the risk document.
Capture More Information With Custom Fields
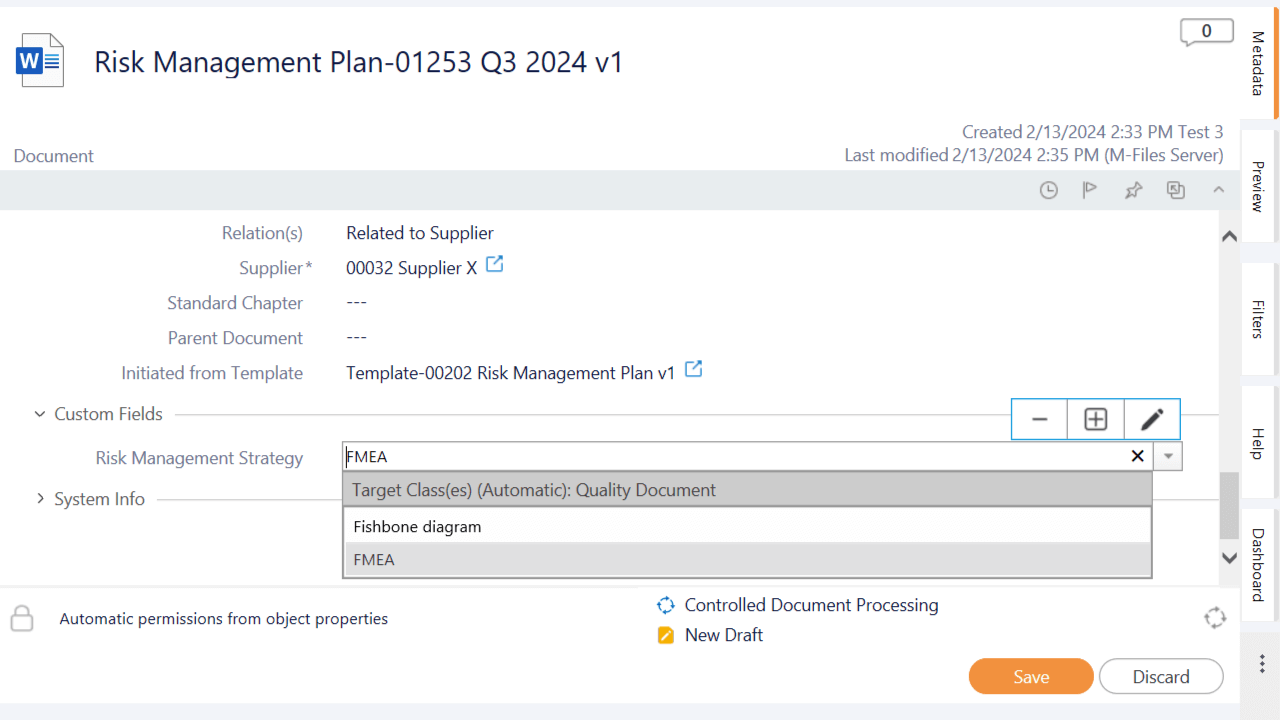
Create custom fields to capture more information about the risk management process.
Add additional data to risk documents to enhance information for strategic monitoring and statistic calculations.
Use lists, numbers, dates, and text to add additional information to risk documents. For instance, specify custom fields to indicate the risk strategy, such as FMEA or fishbone.
Search for personalized risk management data across a wide range of documents using search and search filters.
Ensure Regulatory Compliance
Life science companies must have a process for risk management and maintain risk management documentation.
SimplerQMS supports compliance with Life Science requirements, such as ISO 13485:2016, ISO 9001:2015, ISO 14971:2019, GMP, ICH Q9, EU MDR and IVDR, and more.
The system provides the capability to sign documents with electronic signatures in a manner compliant with FDA 21 CFR Part 11 and EU Annex 11.
SimplerQMS is a fully validated QMS software solution according to GAMP 5 with continuous re-validation for ongoing compliance.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Complete QMS Software for Life Sciences
Document Management
Maintain control over all quality and regulatory documentation with centralized and secure management.
Change Management
Implement changes in your QMS effectively without compromising on structure or compliance.
Form Management
Streamline the creation and utilization of standardized forms and templates across your organization.
Audit Management
Reduce the effort and time needed to prepare for and undergo audits with efficient audit management tools.
Supplier Management
Manage supplier processes, from initial qualification and selection through ongoing monitoring.
CAPA Management
Manage CAPA activities seamlessly from identification to resolution and reporting.
Frequently Asked Questions
What Is Risk Management in Life Science?
Risk management in the Life Science industry involves systematically identifying, assessing, and mitigating potential risks. The potential risks are associated with various aspects of research, development, manufacturing, and distribution of pharmaceuticals, medical devices, and other healthcare products and processes.
Risk management ensures product safety, efficacy, and compliance with regulatory requirements. Risk management aims to proactively address risks, employing strategies and controls to minimize adverse impacts on product quality, patient safety, and overall business operations.
Risk Management is a requirement for Life Science companies. Risk management is specified in standards, regulations, and guidelines, such as ISO 9001:2015, ISO 13485:2016, MDR and IVDR, and ICH Q9 and Q10, among others.
What Are the Benefits of QMS Software With Risk Management Capabilities?
QMS software with integrated risk management capabilities offers several benefits:
- Improved Product Safety and Efficacy: Potential risks across the product development lifecycle are identified and mitigated, ensuring that pharmaceuticals, medical devices, and healthcare products maintain high and uniform standards of safety and efficacy.
- Compliance with Regulatory Requirements: The proactive nature of risk management supports aligning quality processes with stringent regulatory requirements. By identifying and addressing potential risks early on, companies can achieve compliance, avoid regulatory penalties, and adhere to quality and safety measures.
- Risk Mitigation for Business Operations: Effective risk management offers a proactive approach to addressing risks that could impact broader business operations. Risk mitigation strategies and controls help companies minimize disruptions, ensuring the continuity and stability of operations in the face of potential challenges.
How Much Does QMS Software With Risk Management Cost?
SimplerQMS risk management module is part of an all-in-one eQMS platform, which includes all QMS modules, system implementation, user training, ongoing support, validation, cloud hosting, and more.
The cost of the SimplerQMS solution is based on the number of licenses you acquire. SimplerQMS includes all features and services in its subscription price, so there are no additional costs.
For detailed information on license types, features, and included services, refer to our pricing page.
How Much Time Does It Take to Implement an eQMS With Risk Management?
The SimplerQMS complete solution, including the risk management module, can take five to six weeks to be implemented.
SimplerQMS employs a flexible implementation process structured in phases. Each phase involves the deployment of distinct QMS modules. The order of module sequence is adaptable, allowing customization to align with the specific operational requirements of each customer.
For instance, if proactive risk identification and mitigation are a priority for your organization, the risk management module can be deployed early in the implementation process, following the necessary modules for optimal functioning.
During every phase, the SimplerQMS team collaborates closely with customers to ensure the precise configuration of the system. We provide support for all implementation phases at no additional cost.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.