The European Union In Vitro Diagnostic Regulation (EU IVDR) is the European law concerning placing in vitro diagnostic medical devices for human use and their accessories on the market.
The IVDR was introduced in May 2017 to improve the safety and efficacy of in vitro diagnostic medical devices available in the EU market.
A comprehensive quality management system (QMS) supports manufacturers of in vitro diagnostic medical devices to comply with the IVDR requirements regarding quality.
This article will discuss IVDR, its purpose, the rules for the device classification, some of the QMS requirements, and the role of electronic QMS (eQMS) in supporting compliance with IVDR. We will also give examples that illustrate the important role played by eQMS.
SimplerQMS has helped dozens of Life Science companies transition to a modern electronic QMS system and realize its associated benefits, including easier regulatory compliance. If you want to learn more about how an eQMS can help you streamline quality management processes, book a personalized demo.
Learn more about EU IVDR in the following topics:
- What is EU IVDR?
- What Is The Purpose of EU IVDR?
- IVDR Device Classification
- EU IVDR QMS Requirements
- Frequently Asked Questions About EU IVDR
- The Role of QMS Software in Supporting Compliance With EU IVDR
What is EU IVDR?
The EU IVDR stands for European Union In Vitro Diagnostic Regulations and is the most common way of referring to Regulation (EU) 2017/746.
The IVDR specifies the requirements for placing in vitro diagnostic medical devices and their accessories on the EU market.
It defines an in vitro medical device as any device used in vitro to examine samples derived from the human body to provide relevant information for diagnostic purposes. These can be a reagent, product, calibrator, control material, software, or system used alone or in combination.
This regulation was introduced in May 2017 and has a transition period for manufacturers to comply with the requirements until May 2025 to May 2028, depending on device classification.
Manufacturers of medical devices that wish to place in vitro diagnostic medical devices on the EU market must comply with EU IVDR requirements.
EU IVDR vs. IVDD
EU IVDR replaced Directive 98/79/EC, also known as the In Vitro Diagnostic Directive (IVDD).
Since the IVDD was first issued in 1998, some of its requirements are not aligned with current knowledge of in vitro medical devices.
The IVDD and IVDR share the same basic regulatory process. But IVDR specifies comprehensive requirements for in vitro diagnostic medical devices.
The IVDR adds requirements, such as greater attention to risk management, device classification, reporting to authorities, device traceability, post-market surveillance system, and so on.
Lastly, the IVDD is a directive, and manufacturers could choose to comply with its requirements. However, IVDR is a regulation, so compliance with requirements is mandatory.
EU IVDR vs. EU MDR
Together with IVDR, the European Parliament introduced the Regulation (EU) 2017/745, known as the Medical Device Regulation (MDR).
The MDR specifies requirements to place medical devices in the EU market. It refers to a broad category of medical devices. However, it does not apply to in vitro diagnostic devices, being the requirements for these specified in the IVDR.
So, medical device manufacturers in the EU must comply with the requirements specified in MDR or IVDR, depending on their device type.
The IVDR relates to MDR in many aspects, for instance, concerning the identification and traceability of devices, conformity assessment by Notified Bodies, QMS requirements, and risk management.
The difference between IVDR and MDR can be observed in the clinical evidence needed to demonstrate conformity. For IVDR, clinical evidence, performance evaluation, and performance studies are required. While in MDR, manufacturers must conduct and document clinical evaluation and clinical investigation.
If you want to know more about MDR and how an eQMS can help you achieve compliance with it, read our article on the EU MDR Quality Management System, and the role of an eQMS.
What Is The Purpose of EU IVDR?
The purpose of EU IVDR is to help ensure in vitro diagnostic medical devices placed on the EU market are safe and effective.
The IVDR is part of the overall EU regulation for medical devices. It specifies the requirements manufacturers must comply with in order to sell and market an in vitro diagnostic medical device in Europe.
Compliance and certification to this regulation allow companies to commercialize their products across the European Economic Area. The only exception is devices used exclusively for research, where IVDR does not apply.
For instance, a company that manufactures self-testing devices, such as pregnancy tests or HIV detection assays, must comply with the IVDR and be certified before selling in the EU.
IVDR Device Classification
The IVDR classification rules in Annex VIII specify four classes for in vitro diagnostic devices: A, B, C, or D. These classes are based on the intended purpose of the devices and potential risk for end-users.
- Class A: Have low patient and public health risks. They include laboratory instruments, buffer solutions, and specimen receptacles.
- Class B: Have moderate patient risk and low public health risk. They include pregnancy tests and glucose level tests.
- Class C: Have high patient risk and moderate public health risk. They include sexually transmitted infection tests, cancer markers, and human genetic tests.
- Class D: Have both high patient risk and high public health risk. They include blood groupings, such as ABO and the Rhesus system.
Under the IVDR, only class A non-sterile devices can be self-certified by the manufacturer.
Before marketing, in vitro diagnostic devices from the remaining classes must be audited and certified by a Notified Body.
EU IVDR QMS Requirements
The IVDR has several articles to cover all requirements for in vitro diagnostic medical devices. Among them, the regulation specifies that manufacturers who wish to place their devices on the EU market must implement and maintain a Quality Management System (QMS).
The following topics are some examples of where an efficient QMS can help companies achieve compliance with the IVDR QMS requirements:
- General safety and performance requirements
- Technical documentation
- Responsibility of management
- Resource management
- Risk management
- Post-market surveillance
- Unique Device Identification (UDI)
- Reporting to authorities
- CAPA management
NOTE
This article will discuss some of the requirements present in the IVDR. The information here is just a part of the regulation and has educational purposes only. Please always refer to In Vitro Diagnostic Regulation (IVDR) for official information.
1. General Safety and Performance Requirements
Manufacturers must ensure their devices achieve the performance intended during normal conditions of use. For that, devices need to be designed and manufactured to be suitable for the proposed safety and performance.
To comply with this requirement, design, development, and manufacturing documentation must be planned and controlled. As a result, in this stage, a high volume of documents is generated.
SimplerQMS Design Control Software facilitates document creation with a document template package based on Life Science requirements and pre-configured workflows to guide you through the process.
The system offers Life Science companies the capability of storing data in a cloud-based repository, defining access levels to documents, relating documents to products, escalating issues to CAPA, and more.
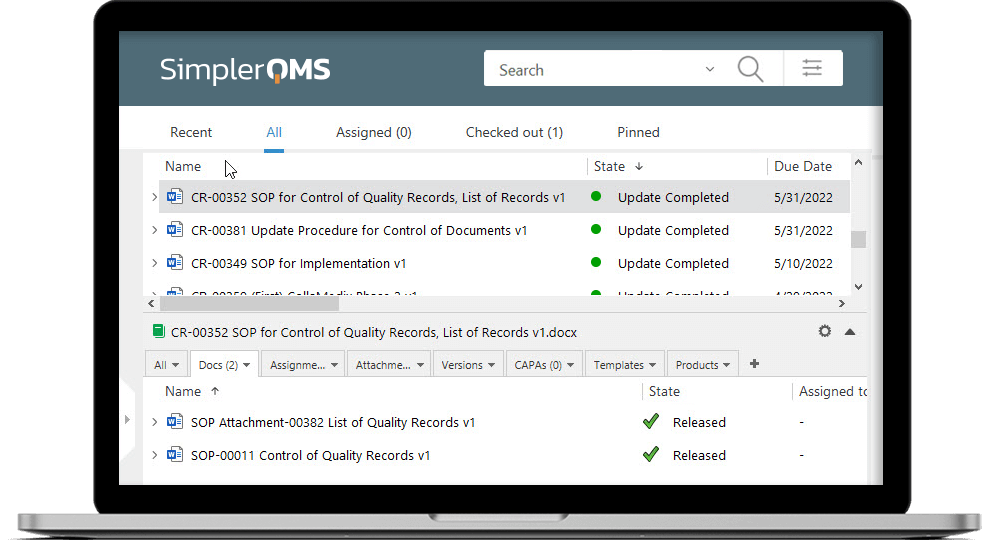
2. Technical Documentation
The technical documentation must be presented in a clear, organized, readily searchable, and precise manner.
It includes several documents, such as device description and specification, reference to previous and similar generations of the device, labels used on the device and its packaging, and so on.
Manufacturers can use Document Control Software, such as the one integrated into the SimplerQMS solution, to help streamline the process of reviewing and approving documents. This core QMS module helps keep documents audit-ready while accurately controlling a high volume of data.
To know more about how to handle documents, read our article about medical device document control.
3. Responsibility of Management
Among many responsibilities, top management must communicate the importance of an effective QMS and the regulatory requirements to all employees. Management will establish the quality policy and objectives of the company.
It is the top management’s responsibility to ensure the effective implementation of the QMS to achieve compliance with IVDR quality systems requirements.
Using an electronic QMS solution like SimplerQMS, you benefit from a single cloud-based system that allows easy access to all QMS data.
The system automatically creates a record of all activities inside the software and provides a time-stamped audit trail. You can easily search and retrieve documents, create customizable document status views, and export data for further analysis.
4. Resource Management
Manufacturers must have a QMS to address resource management to provide adequate resources for conformity assessment activities.
Resource management includes infrastructure, workforce, and suppliers.
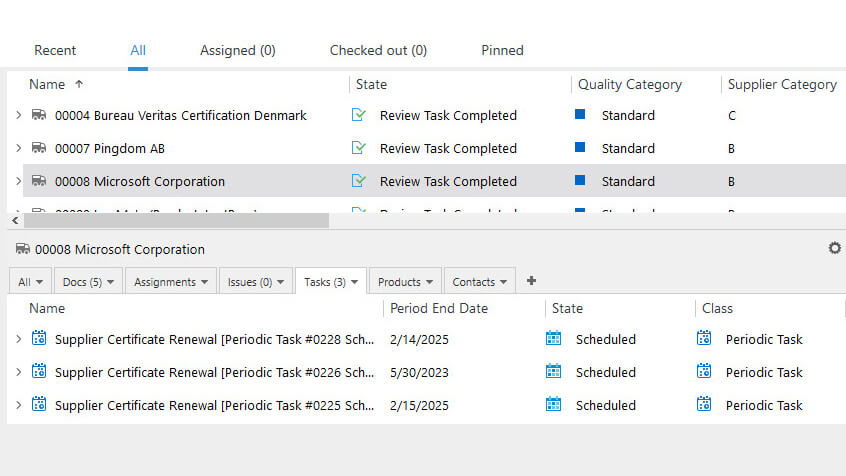
For instance, an important infrastructure element is an equipment used in manufacturing the devices. This equipment needs to be adequately maintained to prevent any issues that can impact product safety and quality.
SimplerQMS provides equipment management software to simplify equipment calibration and maintenance tasks. You can create periodic calibration or review tasks, monitor equipment status, set up automatic reminders of actions before due dates, relate specific products to specific equipment, and more.
Another important requirement is having a trained workforce with the necessary skills to perform their functions efficiently. For example, the employees must know the related procedure to use specific equipment, ensuring the tasks are done consistently.
Life Science companies must have a training plan to ensure all employees are competent to perform their specific activities. Training management is one of the core QMS processes in SimplerQMS and helps companies streamline the training process with learning rules, training groups, task assignments, and quizzes.
Regarding suppliers, implementing an eQMS can help medical device companies manage and monitor their suppliers more effectively. SimplerQMS Supplier Quality Management Software allows you to create and maintain Approved Supplier List, schedule supplier audits, and set up automatic reminders for tasks before due dates.
Read our article on the medical device supplier management process to learn more.
5. Risk Management
In vitro diagnostic devices need to be safe and effective for end-users. So, mitigating risks as far as possible is required without adversely affecting the benefit-risk ratio.
The QMS must have risk identification, assessment, and mitigation procedures throughout an in vitro diagnostic device lifecycle.
A complete eQMS, like SimplerQMS, support you with risk management documentation management. You can relate risk documents to products, components, suppliers, customers, equipment, etc. Easily export data and create a traceability matrix and improve risk mitigation.
6. Post-Market Surveillance
Post-market surveillance (PMS) for medical devices is a process by which manufacturers assess the safety and efficacy of their in vitro diagnostic devices in the market.
This requires manufacturers actively gather information regarding their product after it has been sold. Information can be in the form of customer complaints, a literature search on similar devices, and an evaluation of competitors’ issues.
Any quality events need to be addressed within a proper time frame.
For instance, a company received customer complaints about the packaging of self-test pregnancy kits. Customers have complained that the new packaging is hard to open. Such a complaint is less severe and could be addressed within 10 to 15 days.
On the other hand, several hospitals have complained that some batches of COVID-19 kits have insufficient reagents. These complaints are critical and could be addressed within a day or two.
SimplerQMS is a comprehensive eQMS that can help you streamline your complaint management workflow.
The system offers features such as document creation using a template package based on Life Science requirements, relating complaint documents to customers or products, setting reminders for task due dates, escalating issues to CAPA, etc.
To learn more check out our article on medical device complaint handling processes.
7. Unique Device Identification
The Unique Device Identification (UDI) is an exclusive numeric or alphanumeric code placed on every medical device in the market, including in vitro diagnostic devices.
The UDI is essential for tracing legal devices that are in conformity and certified to IVDR requirements.
The European Database on Medical Devices (EUDAMED) stores the UDI list, where are all crucial information about the device, such as manufacturers’ details, device risk class, market status, certificates, and more.
8. Reporting to Authorities
According to IVDR, the QMS must have proper procedures for handling communications with competent authorities, Notified Bodies, customers, and other stakeholders.
Regarding reporting to authorities, Life Science companies must summarize the results and conclusions of the analyses of the PMS data and report them together with a rationale and description of any corrective and preventive actions taken.
SimplerQMS offers a reporting functionality where you can justify actions taken and summarize reports. This feature can be enabled or disabled depending on your compliance needs to streamline the workflow.
9. CAPA Management
The QMS should have a robust system for managing corrective and preventive actions (CAPAs) regarding quality events, helping to justify the actions taken and verify their effectiveness.
SimplerQMS supports a comprehensive CAPA management software that can track and manage CAPAs to enforce continuous improvement and resolve issues in a timely manner.
The system offers companies best-practice document templates based on Life Science requirements for complaints, non-conformances, and CAPAs. You can streamline processes with our pre-configured workflows while the system automatically records all actions taken in a time-stamped audit trail.
To learn more about the CAPA process, read our article on the CAPA management process in the medical device industry.
Frequently Asked Questions About EU IVDR
What Does EU IVDR Replace?
The European Union’s In Vitro Diagnostic Regulations (EU IVDR) replaces the In Vitro Diagnostic Directive (IVDD).
The IVDR was implemented in May 2017 and has a transition period until May 2025 to May 2018, depending on the in vitro diagnostic device classification.
Is ISO 13485:2016 Required for EU IVDR?
The ISO 13485:2016 standard is not required for IVDR. However, many manufacturers of in vitro diagnostic devices usually comply with this standard since following it presumes compliance with QMS requirements presented in the IVDR.
What Is the Main Difference Between EU MDR and EU IVDR?
The main difference between EU MDR and EU IVDR is that MDR requirements apply to all medical device types for human use, except in vitro diagnostic devices.
The IVDR is specific for in vitro diagnostic medical devices placed on the EU market.
What Is the Main Difference Between EU IVDR And IVDD?
The main difference between EU IVDR and IVDD is that IVDD is a directive with voluntary compliance, while IVDR is a regulation and so mandatory for every member state in the EU.
IVDR and IVDD have basic requirements in common, but IVDR is a more comprehensive regulation.
Which Countries Does IVDR Apply To?
The EU IVDR applies to all countries in the European Economic Area.
The Role of QMS Software in Supporting Compliance With EU IVDR
Manual paper-based or hybrid QMS have been used traditionally by companies to comply with Life Science regulations and standards requirements. These manual QMS can be successfully used when the company is small and has the necessary resources for the paperwork.
Nevertheless, when considering current regulatory requirements, such as those presented in IVDR, it is evident that manual paper-based and hybrid QMS are becoming increasingly insufficient. These systems bring their own challenges, for instance, physical storage problems, security issues, lost files, and so on.
So, implementing modern, reliable, and efficient QMS software for medical devices, such as SimplerQMS, facilitates quality management. An eQMS provides many features to help achieve compliance with IVDR requirements, streamline QMS processes, enhance efficiency, and lower costs.
Some of the best QMS software solutions for life sciences provide companies with robust and comprehensive QMS modules for document control, change management, employee training, CAPA and non-conformances management, customer complaints handling, supplier management, internal and external audits management, and much more.
SimplerQMS is a state-of-the-art document management system. The system is fully validated according to GAMP5 with monthly re-validation and FDA 21 CFR Part 11 and EU Annex 11 compliant regarding electronic signatures. Our solution covers all Life Science QMS modules in one single place.
Download our eQMS Business Case template to understand the investment in quality management software. The template will assist you in quantifying the investment in an eQMS and help you present it to your company’s decision-makers.
Final Thoughts
One of the most regulated industries in Life Science is medical devices. Due to the vital role medical devices play in patient care, including in vitro diagnostics, this is justifiable.
For instance, the European Centre for Disease Prevention and Control states that within the first two years of COVID-19, over 100 million cases were reported in Europe alone.
These high quantities indicate the number of diagnostic tests conducted across the EU. So, there is a need for strict control by international regulatory authorities.
To place an in vitro diagnostics medical device in the EU market, companies must comply with the requirements presented in the EU IVDR, including QMS requirements.
Many manufacturers choose to achieve compliance with ISO 13485:2016 standard first, as it is presumed to conform with IVDR requirements regarding QMS.
Traditional paper-based or hybrid QMS may still be used at small-sized organizations with sufficient personnel and resources. However, companies are now transitioning to modern and highly efficient eQMS solutions that will streamline the entire QMS process.
If you are interested in implementing an eQMS solution and simplifying achieving compliance with EU IVDR, book a demo of SimplerQMS and talk to our quality solution experts today.