Medical device complaint handling refers to the systematic process by which manufacturers of medical devices receive, review, and address complaints related to their products.
The medical device complaint handling process is a mandatory requirement for medical device manufacturers under various regulatory frameworks, including ISO 13485:2016, 21 CFR Part 820, and the European Medical Device Regulation (MDR).
All these regulatory frameworks emphasize the importance of establishing and maintaining a robust complaint handling system to ensure patient safety, product quality, and regulatory compliance.
This article discusses the entire medical device complaint handling process, including what complaints are, complaint types and examples, and the requirements of the complaint handling process. It also explains the role of the medical device complaint handling software solution.
The complexities of medical device complaint handling highlight the need for QMS software, which provides a centralized and efficient solution to streamline and improve the customer complaint process.
SimplerQMS provides fully validated eQMS software tailored to the unique needs of medical device companies. You can book a free demo and talk to our specialists to see how our software can improve your quality management processes, including the handling of medical device complaints.
This article covers the following topics:
- What Is a Complaint?
- What Are Medical Device Complaint Examples?
- What Are the Different Types of Medical Device Complaints?
- What Are the Requirements for Device Complaint Handling?
- What Is the Medical Device Complaint Handling Process?
- What Are the Benefits of a Well-Designed Complaint Handling Process?
- What Is the Role of Medical Device Complaint Handling Software?
What Is a Complaint?
A complaint is an expression of dissatisfaction made by a customer or client regarding a product, service, or process, typically due to a perceived failure to meet their expectations or a problem encountered.
Below is the definition of complaint according to some requirements that outline complaint handling processes.
- ISO 13485:2016: Complaints are any written, electronic, or oral communication that claims deficiencies related to the identity, quality, durability, reliability, usability, safety, or performance of a medical device or related to a service that affects the performance of such medical devices.
- FDA 21 CFR Part 820: Complaint means any written, electronic, or oral communication that declares deficiencies related to the identity, quality, durability, reliability, safety, effectiveness, or performance of a device after it is released for distribution.
What Is the Difference Between Complaints and Feedback?
The main difference between complaints and feedback lies in their nature and intent.
Complaints are specific expressions of dissatisfaction about a product, service, or experience, often expecting a resolution or response. An example of a complaint is a phone call about a product that is not functioning according to the specifications.
Feedback, on the other hand, is more general and can be positive or negative. Feedback offers insights or opinions to help improve a product, service, or process without necessarily seeking direct resolution of a specific issue. An example of feedback can be an email to demonstrate appreciation for a new product in the market when compared to similar existing products or a survey response detailing negative aspects of a service that could be enhanced.
What Are the Sources of Complaints?
The sources of complaints include multiple communication channels that a company utilizes to interact with its customers.
Below are examples of sources of customer complaints.
- Emails: Complaints received through customer service or company email addresses.
- Phone Calls: Issues reported via call centers or customer support lines.
- Social Media: Complaints are made on social media platforms like LinkedIn, Facebook, Instagram, and others.
- Live Chat: Issues communicated through website live chat.
- Feedback Forms: Complaints are submitted through online or physical feedback forms.
- Text Messages/SMS: Customer grievances are sent via text messaging service or SMS (Short Message Service).
- Company Website: Complaints are submitted through contact forms or portals on the website.
- Direct Mail: Written complaints sent through postal mail.
- Online Review Platforms: Negative reviews or complaints are posted on review platforms like G2 Crowd, Trustpilot, Capterra, Google Reviews, and others.
- In-Person: Direct complaints made at the company’s physical locations.
What Are Medical Device Complaint Examples?
Realistic medical device complaint examples are given below.
- Device Malfunction: A pacemaker is not working as intended, leading to health concerns.
- Software Issues: Glitches in the software of a diagnostic imaging device cause inaccurate results.
- Product Durability: A prosthetic limb breaking or wearing out prematurely.
- Adverse Reactions: Patients experiencing unexpected side effects from using a medical device, like skin irritation from an adhesive in a wearable monitor.
- Inaccuracy: A blood glucose meter provides inaccurate readings.
- Usability Issues: Difficulty in operating a portable dialysis machine due to complex controls.
- Sterility Concerns: Reports of a surgical instrument pack not being properly sterilized.
- Battery Life Problems: Short battery life in a portable oxygen concentrator.
- Incorrect Labeling: A medical device with labels that have incorrect or incomplete usage instructions.
- Delivery Delays: Late delivery of critical medical devices like ventilators to healthcare facilities.
What Are the Different Types of Medical Device Complaints?
Medical device complaints can be grouped into two types: incident-driven and review-driven.
Incident-Driven Complaints
Incident-driven complaints arise due to specific incidents related to the use or performance of the medical device. These complaints demand immediate attention from the company.
Examples of incident-driven complaints are listed below.
- Device malfunctions: The device delivered the wrong medication or dosage, causing the patient to suffer adverse effects.
- Adverse reactions: A patient complains that they developed a rash after using a new device.
- Equipment failures: Defibrillators with electrical problems that could prevent them from delivering a shock.
- Issues leading to inaccurate results or patient harm: A laboratory technician reports that a blood glucose meter is giving inaccurate readings, which could lead to patients receiving incorrect treatment.
Review-Driven Complaints
Review-driven complaints are identified by gathering and analyzing any reported issues regarding the medical device. Review-driven complaints represent past issues and are reviewed periodically.
These complaints include information from various sources, such as incident reports, trend reports, technical literature, distributors’ and importers’ feedback, and customer online reviews.
Review-driven complaints are a valuable source of information for post-market surveillance (PMS), as they provide real-world evidence of how medical devices are being used and how they are performing in clinical practice.
Examples of review-driven complaints are listed below.
- Device malfunctions: Multiple reviews on a retailer’s website mention that a particular blood pressure monitor consistently provides inaccurate readings.
- Device durability: A customer on an online forum complains that a particular medical device broke after a short period of use.
- Software issues: Multiple reviews on an app store complain that a particular software application frequently crashes or freezes, disrupting their workflow or causing data loss.
- Equipment failures: A routine maintenance report reveals that an X-ray machine is not producing clear images.
What Are the Requirements for Device Complaint Handling?
The requirements for device complaint handling include regulations and standards, such as US FDA 21 CFR Part 820, EU MDR and IVDR, and ISO 13485:2016, among others.
NOTE
This section will discuss some of the requirements that specify medical device complaint handling. While this list provides a comprehensive overview, it is not exhaustive. For official information, always refer to the requirements applicable to your company.
ISO 13485:2016
The ISO 13485:2016 is an international standard for quality management systems in the medical device industry.
The standard Section 8.2.2 states that medical device companies must establish and document procedures for handling complaints promptly and in compliance with all relevant regulatory requirements.
These procedures must outline the minimum requirements and responsibilities for:
- Receiving and documenting complaints.
- Assessing whether feedback qualifies as a complaint.
- Investigating complaints thoroughly.
- Determining if regulatory authorities should be notified.
- Managing complaint-related products.
- Deciding whether corrective actions are necessary.
Any corrective action resulting from the complaint handling process should be documented.
The medical device complaint handling process requires appropriate procedures and controls, but other processes are equally crucial for establishing an ISO 13485:2016 compliant Quality Management System (QMS), as detailed in our dedicated guide.
21 CFR Part 820
The 21 CFR Part 820 is a regulation issued by the United States Food and Drug Administration (FDA) that establishes the quality system regulation and the Current Good Manufacturing Practice (cGMP) for medical device manufacturers.
In Section 21 CFR 820.198, the regulation specifies that manufacturers must maintain complaint files and establish procedures for receiving, reviewing, and evaluating complaints through a designated unit.
These procedures must ensure that all complaints are processed in a consistent and timely manner.
A comprehensive record of the complaint investigation should include the following:
- The name of the medical device involved
- The date the complaint was received
- Any unique device identifier (UDI) or universal product code (UPC), along with any other relevant device identification codes or control numbers
- The name, address, and phone number of the individual who filed the complaint
- A detailed description of the nature and specifics of the complaint
- The dates and outcomes of the investigation
- Any corrective actions taken in response to the complaint
- A copy of the response provided to the complainant
In instances where no investigation is conducted, the company must maintain a documented record specifying the rationale and responsible person behind the decision.
Complaints are evaluated to determine whether they represent an event that is required to be reported to the FDA under the regulation 21 CFR Part 803, Medical Device Reporting.
To delve into a comprehensive overview of all the requirements outlined in 21 CFR Part 820 quality system regulation, refer to our dedicated article.
21 CFR Part 803
The FDA 21 CFR Part 803 is a regulation that establishes requirements for medical device reporting. Complaints need to be reported to the FDA under 21 CFR Part 803 when they are related to events of deaths and serious injuries.
The purpose of the regulation is to ensure that the FDA is aware of any serious injuries or deaths associated with medical devices.
The regulation Section 21 CFR 803.18(d) states that device companies must implement and maintain procedures for recording device complaints.
To ensure comprehensive and informative reporting, below are some examples of information that must be included in the medical device reporting:
- Patient information: name or identifier, age at the time of event, date of birth, gender, and weight.
- Patient medical history: pre-existing medical conditions, allergies, and current medications.
- Detailed event description: provide a comprehensive description of the adverse event or product problem.
- Outcomes attributed to the event: consequences associated with the adverse event, such as death, serious injury, or other health complications.
- Relevant tests and laboratory data: dates and results of relevant clinical tests or laboratory data conducted in relation to the event.
- Device information: device name, model number, and device lot number or serial number.
- Manufacturer information: name, address, and contact person.
MDR and IVDR
The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) are two regulations in the European Union that outline regulatory requirements for medical devices and in vitro diagnostic medical devices, respectively.
These regulations aim to ensure that devices meet the requirements of safety, performance, and quality.
According to Article 87, manufacturers must submit vigilance reports. Vigilance reporting is the process of collecting, evaluating, and reporting incidents and field safety corrective actions related to medical devices.
All medical device incidents that result in death, serious injury, or an unexpected deterioration in a patient’s health must be reported to the relevant authorities.
In the MDR and IVDR Annex III, feedback and complaints are integral parts of the post-market surveillance plan.
Post-market surveillance (PMS) is the process of monitoring the safety and performance of medical devices after they have been placed on the market to identify and address any potential safety issues.
The post-market surveillance plan must specify the effective and appropriate methods and tools for investigating complaints.
The following are examples of PMS data that should be collected and addressed according to MDR and IVDR.
- Information concerning serious incidents.
- Information from field safety corrective actions.
- Records of non-serious incidents.
- Data on any undesirable side effects.
- Information from trend reporting.
- Information from relevant specialist or technical literature, databases, and registers related to the medical device.
- All information is provided by users, distributors, and importers in the form of complaints and feedback.
- Publicly available information about similar medical devices.
- Information from periodic safety update reports (PSURs).
What Is the Medical Device Complaint Handling Process?
The medical device complaint handling process is a systematic approach to receiving, evaluating, investigating, and responding to complaints regarding medical device products.
The steps involved in the medical device complaint handling process are listed below.
- Receiving the customer complaint
- Registering the complaint
- Reporting medical device complaints (if applicable)
- Investigating the complaint
- Closing the complaint
The steps outlined in the medical device compliant handling flowchart provide a general framework for the complaint handling process.
The following sections provide a more detailed explanation of each step, accompanied by examples of how SimplerQMS streamlines the complaint handling process.
1. Receive the Customer Complaint
The complaint-handling process begins with receiving and acknowledging the customer’s complaint.
Companies should provide customers with multiple communication channels to submit complaints, such as phone, email, in-person, and others.
All employees in the company can receive complaints and should be aware of the procedure to forward them to the appropriate person or team. Complaints can be forwarded to supervisors, managers, or a specific department responsible for addressing customer complaints.
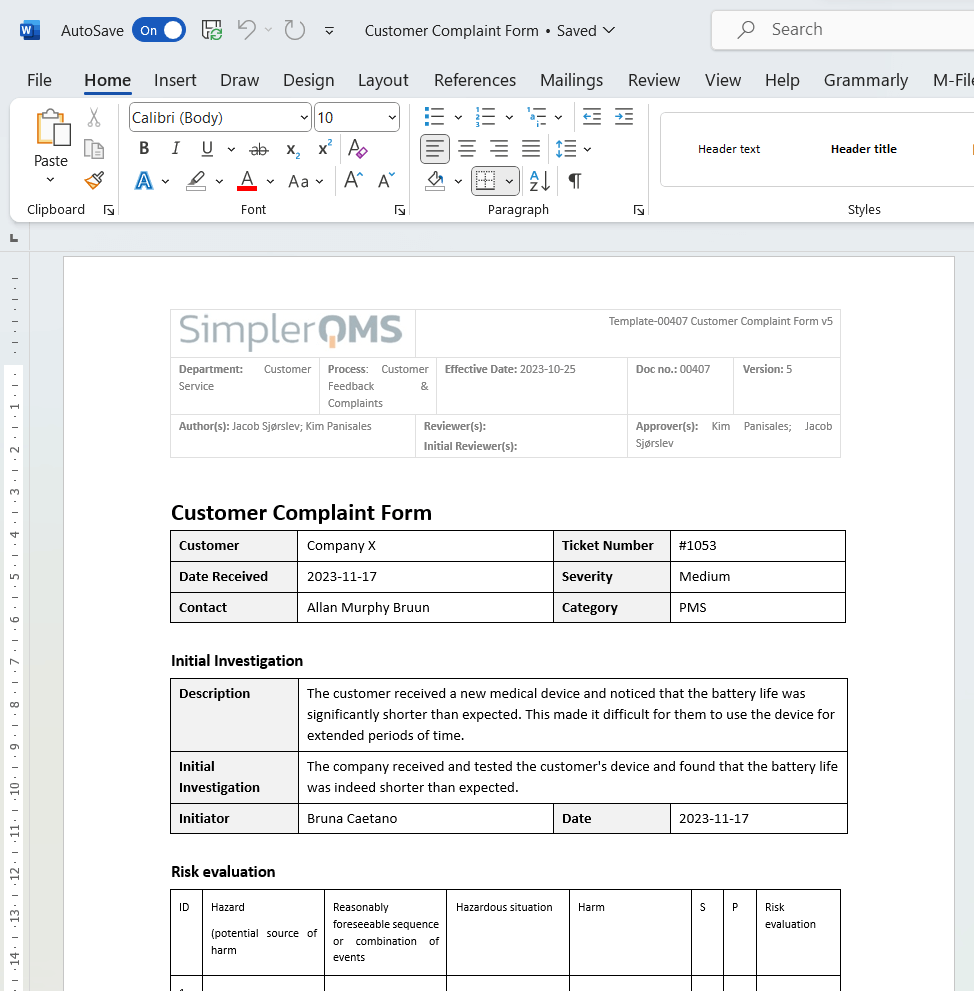
It is important to accurately document the customer’s complaint. The complaint form document must include the customer’s name, contact information, a description of the issue, and any relevant details as references for further investigation and resolution.
2. Register the Complaint
Registering a medical device complaint involves creating a formal record of the complaint and assigning it a unique number in the system, such as a customer complaint form.
The complaint record should capture details about the issue, including the date and time of the complaint, complaint description, contact information, initial investigation, and more.
All complaints must be recorded promptly within the specified timeframe, for example, 24 hours after receipt during business hours.
SimplerQMS software provides customizable forms and templates to facilitate the creation of customer complaint forms, ensuring that all relevant information is captured and organized.
Our system provides a centralized repository for customer complaints and related documents, ensuring that all relevant information is readily accessible to authorized personnel.
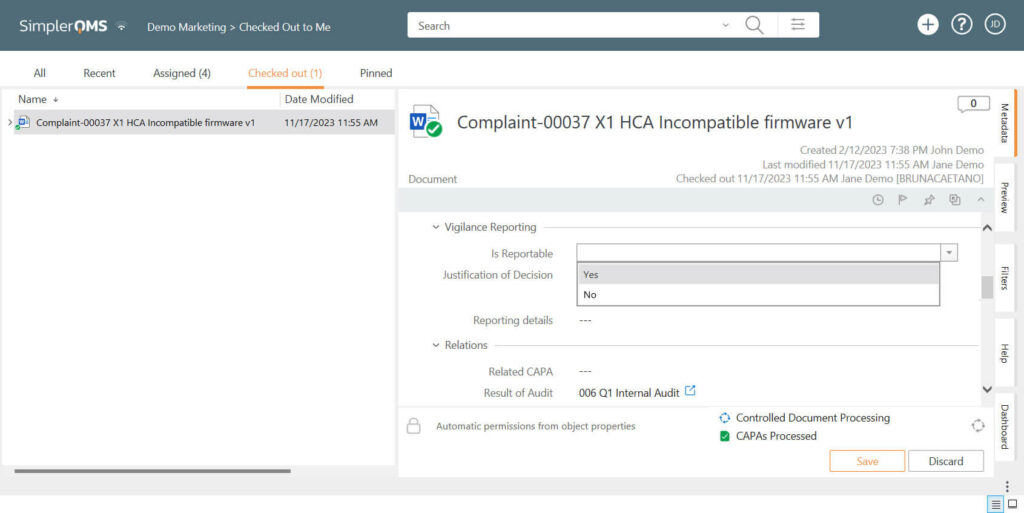
3. Reporting Medical Device Complaints (If Applicable)
According to 21 CFR Part 803 and EU MDR and IVDR, for complaints that raise potential safety concerns, such as deaths and serious injuries, a medical device vigilance report must be prepared and submitted to the regulatory authorities.
SimplerQMS allows you to specify whether the complaints are reportable or non-reportable.
4. Investigate the Complaint
A thorough investigation of the complaint should be performed to determine the root cause of the issue.
The investigative stage includes collecting all relevant information related to the complaint, including device documentation, patient information, and complaint details.
Once the relevant information is assembled, the next step involves a meticulous analysis of the gathered data to determine the severity and risks of the incident.
Quality assurance personnel then determine whether corrective and preventive actions (CAPA) are necessary to address the issue and prevent future occurrences. These actions may include design modifications, changes to the manufacturing process, or training updates.
SimplerQMS offers automatic reminders and notifications to ensure that all tasks related to the investigation are completed on time, such as reviewing the complaint details, reviewing previous complaints, examining the affected device, performing device testing, and more.
Complaints can be escalated directly to the CAPA process with all documents linked, ensuring traceability and maintaining a comprehensive record of the investigation and resolution process.
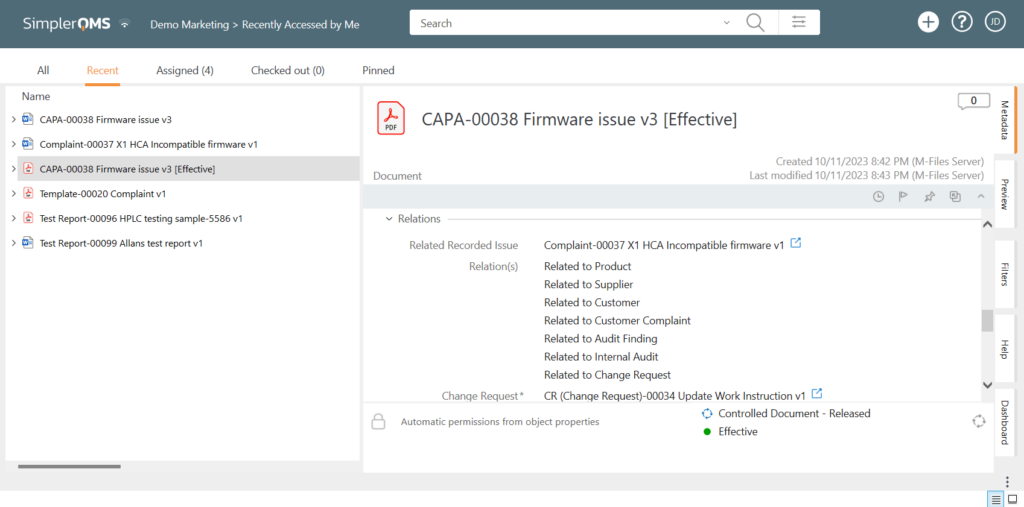
5. Close the Complaint
The complaint is formally closed once the complaint handling process is complete and any necessary corrective and preventive actions have been implemented.
Closing a complaint involves documenting its final status with a concise summary of the resolution process. The findings of the investigation and the actions taken to address the issue and prevent the problem from recurring must also be described.
For regulatory purposes, all relevant records must be properly documented.
SimplerQMS provides a time-stamped audit trail that automatically records every step of the complaint-handling process.
The system records all actions in documents, from initial filing to closure, providing a transparent and traceable history of the complaint.
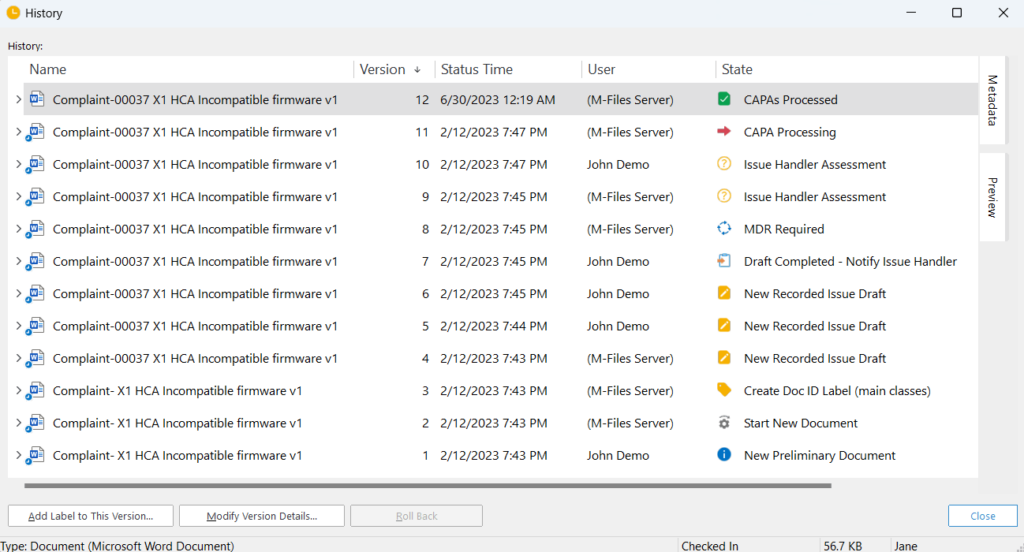
What Are the Benefits of a Well-Designed Complaint Handling Process?
A well-designed complaint handling process offers numerous benefits for the company and customers.
Some benefits of having a well-designed complaint process are the following.
Regulatory Compliance
A well-designed medical device complaint handling process can help companies stay compliant and avoid noncompliance with requirements such as ISO 13485:2016, MDR, and 21 CFR Part 803 and 820.
According to the official Food and Drug Administration (FDA) Data Dashboard, 368 inspection citations were issued between November 2022 and November 2023 related to the 21 CFR Part 820.198 section regarding complaint files.
Regulatory issues involving 21 CFR Part 820.198 are listed below.
- Procedures for receiving, reviewing, and evaluating complaints by a formally designated unit have not been adequately established (240 recurrences).
- Complaints involving the possible failure of a device to meet any of its specifications were not investigated where necessary (40 recurrences).
- Complaint files are not adequately maintained (33 recurrences).
- Records for complaints where no investigation was made do not include the required information (31 recurrences).
- Not all complaints have been reviewed and evaluated to determine whether an investigation is necessary (12 recurrences).
- Complaints representing events that are MDR reportable were not promptly reviewed, evaluated, and investigated by a designated individual (11 recurrences).
- Investigation records of MDR reportable complaints do not include the required information (1 recurrence).
Improved Product Quality
A well-designed complaint handling process can help companies identify issues and prevent similar ones from happening again. These actions lead to improved product quality and safety.
Increased Customer Satisfaction
By promptly and effectively addressing customer complaints, companies can demonstrate their commitment to customer satisfaction.
Customers appreciate it when their complaints or feedback are listened to and corrected or implemented, improving customer loyalty.
When customers know that their complaints will be taken seriously and addressed promptly, they are more likely to feel confident in the company and continue doing business with them, building a stronger brand reputation.
Reduced Costs
Companies can reduce costs associated with customer dissatisfaction and prevent future complaints by analyzing customer complaints and addressing identified gaps in their services or products.
Early detection and correction of product issues can avoid the necessity for costly product recalls, which cause substantial expenses related to product withdrawal, customer notification, and replacement provision.
A well-designed complaint handling process can improve product quality, customer satisfaction, and regulatory compliance. It also makes it easier to use specialized software to streamline complaint management.
What Is the Role of Medical Device Complaint Handling Software?
The role of medical device complaint handling software is to streamline the complaint handling process, offering an efficient and systematic approach to managing and resolving complaints.
Modern eQMS solutions have integrated complaint handling capabilities that provide a centralized platform for managing complaints facilitating collaboration among team members. Additionally, the capability to relate documents for traceability allows users to easily track the history of a complaint.
SimplerQMS is a fully validated eQMS software solution for medical device companies with built-in Complaint Management capabilities.
Our solution streamlines the complaint handling process by automating tasks, providing workflows and centralized access to information, and facilitating collaboration among departments.
The software helps medical device companies comply with a variety of requirements, including ISO 13485:2016, MDR and IVDR, FDA 21 CFR Part 803 and 820, and more.
The SimplerQMS solution supports companies in achieving compliance with regulatory requirements by providing comprehensive QMS process support.
Besides complaint management, SimplerQMS provides support for several other QMS processes, such as document control, change control, training management, design control, nonconformance management, CAPA management, audit management, supplier management, and more.
To assess how an investment in an eQMS solution could benefit your company, download our eQMS Business Case template today.
You can build a stronger argument for implementing an eQMS using our template. Clearly explain to management the practical benefits of SimplerQMS, such as improved efficiency, cost savings, and enhanced compliance efforts.
Final Thoughts
The medical device complaint handling process is a systematic approach employed by companies to efficiently manage and resolve complaints. This process encompasses receiving, reviewing, evaluating, investigating, and addressing customer complaints in a timely and effective manner.
The complex nature of medical device complaint handling processes emphasizes the use of Quality Management System (QMS) software. The software streamlines the customer complaint process.
Modern eQMS solutions combine complaint management features with a centralized platform for easy document management, allowing users to track complaint history and gather data seamlessly.
SimplerQMS is a fully validated eQMS software solution tailored for medical device companies with integrated complaint management capabilities.
If you are interested in learning more about how SimplerQMS can help you streamline complaint handling and other quality management processes, we recommend you book a personalized demo today and talk to our Quality Solution Consultants.