The tough part for the medical device industry is that this landscape of regulations is constantly changing, and therefore, intimidating. The European Union’s Medical Device Regulations (EU MDR) which came into effect on 26 May 2021 after a three-year transition period, is no exception.
The EU MDR (formally, EU MDR 2017/745) replaced the EU’s Medical Device Directive (93/42/EEC) and the EU’s Directive on active implantable medical devices (90/385/EEC).
When we look specifically at the Quality Management System (QMS) requirements, they are detailed in Article 10 (9) of EU MDR. For those companies familiar with the EU’s medical device QMS standard, EN ISO 13485:2016, the resemblance with Article 10(9) will immediately stand out.
In this article, we will take an in-depth look at what EU MDR is all about, the main QMS requirements, as well as the role of QMS software in supporting compliance with EU MDR regulations.
If you are keen on learning more about how a medical device eQMS system such as SimplerQMS can help your organization streamline quality management processes, make compliance with EU MDR easier, and stay ahead of your competitors – book a demo and talk to one of our experts today.
But first, let us take a step back and look at the big picture.
- What is EU MDR?
- What is The Purpose of EU MDR?
- EU MDR QMS Requirements
- The Role of QMS Software in Supporting Compliance with EU MDR
What is EU MDR?
EU MDR stands for European Union Medical Device Regulations. It is a set of regulations governing both the production and distribution of medical devices in the European Union.
Medical device companies that plan to market their devices in the European Union, and require CE Marking, must comply with the regulations outlined in EU MDR.
Considering the importance of safety, quality, and efficacy of medical devices in the lives of end-users, the medical device industry must have in place an effective and efficient QMS. The EU MDR provides details for the overall responsibilities, procedures, and processes for Quality Management Systems.
As a medical device manufacturer, you will need to have a set of EU MDR-compliant systems, documents, and processes that will continually monitor the safety, efficacy, and efficiency of your products. These regulations are pertinent for medical device manufacturers, importers, distributors of medical devices into the EU, and authorized representatives of medical device companies.
EU MDR vs EU MDD
The EU Medical Devices Directive (MDD) was introduced in 1994 to regulate medical devices sold in the European Union. It was replaced by the new regulation, EU MDR that is intended to both improve the safety and performance of medical devices used in Europe and to provide an increased level of protection for patients and users of these devices.
EU MDR continues to have all the requirements mentioned in MDD, with the addition of some new requirements.
The key changes in EU MDR when compared to MDD are as follows.
Stricter Classification of Medical Devices
Medical device manufacturers, particularly those manufacturing devices that are to be used for surgeries, implantation, or other invasive procedures, including related software, must understand that the new classification of medical devices is now stricter.
For instance, medical devices that were under Class I of MDD will now come under Class 2a, at least.
MDR Annex VIII, Section 6 gives a comprehensive description of the new classification rules.
Furthermore, when the software drives a given medical device or plays a crucial role in the use of such a device, it will come under the same class as the device (refer to MDR Annex VIII, Chapter 2, Section 3.3).
Learn more about EU MDR medical device classification in our dedicated article.
Enhanced Traceability
EU MDR has introduced a Unique Device Identification (UDI) system that expedites easier traceability of medical devices.
This system aims to provide several benefits including:
- Reduction of medical errors
- Tracing falsified devices
- Improved purchasing and waste disposal
- Improved post-market safety-related activities and monitoring
The EU MDR’s Unique Device Identification (UDI) system is a distinctive numeric/alphanumeric code associated with a medical device. This UDI helps in the explicit identification of every device in the market. This facilitates their traceability.
Changes in the Quality Management System (QMS)
Under EU MDR regulations in Article 10, the scope of your medical device company’s QMS will now include protocols for clinically evaluating and operating a post-market surveillance (PMS) system.
Additionally, a post-market clinical follow-up (PMCF) is required for every product you manufacture.
Requirements for Notified Bodies
Annex VII (Requirements to be met by Notified Bodies) of EU MDR highlights requirements for the designation of Notified Bodies.
This means that every Notified Body must be set up in accordance with a member state’s national laws. Complete documentation should be available for the legality and the status of such a Notified Body.
These Notified Bodies must also provide information on their ownership.
Need for an Independent Expert Panel
When your company’s medical device comes under Classes IIa, IIb, or III, you will need to involve a Notified Body for conformity assessment. Notified Bodies are required to consult with expert panels before clearing certain high-risk devices.
This is obligatory when a Class III device is intended for implantation.
Improved Transparency
Under EU MDR regulations, information on all medical device products and tests is now in the public domain.
The European Electronic Database for Medical Device Information (EUDAMED) will provide a clear-cut picture of all devices that are available in the EU market.
This will help with transparency and assist coordination between the EU Member States.
Rigorous Clinical Evaluation Requirements
Clinical evaluation requirements are now tightened up under EU MDR regulations.
These include clinical data collection and the organization of clinical studies.
Implantable medical devices and Class III devices are required to undergo clinical trials before being marketed. For certain Class IIb and Class III devices that are used for drug management (within/outside the body), the medical device company has the option of consulting specified EU experts regarding a clinical development plan.
What is The Purpose of EU MDR?
The purpose of EU MDR regulations is to improve the safety and performance of medical devices used in the European Union. With the implementation of these regulations, your company and the regulatory agencies will ensure that there is enhanced protection for the end-users of your company’s medical devices.
For instance, if you doubt how to classify tracheotomy tubes and blood bags that you manufacture and intend to sell in the EU, the new regulations clearly state that blood bags come under Class IIb (Medium/High risk) and tracheotomy tubes will come under Class IIa (Medium Risk).
Another example there are reports of drug-coated stents causing serious health hazards in patients. On investigation, these stents have been identified as counterfeit. Based on their UDI (Unique Device Identification) number, it becomes easier for regulatory authorities to trace them back to the manufacturer.
When it comes to complying with EU MDR regulations, the use of traditional paper-based or hybrid QMS systems comes with its own set of challenges – storage, security, human errors, traceability, and costs, to name a few.
On the other hand, an electronic QMS made for medical devices such as SimplerQMS helps you manage and control all your quality processes in one place. From design and development to post-market surveillance, our medical device quality management software is equipped with the necessary tools and features to support your EU MDR compliance journey.
EU MDR QMS Requirements
As mentioned earlier, the QMS requirements for your medical device company are given in detail in Article 10 (9) of the current EU MDR.
Let us now deep dive into the indispensable aspects that your medical device quality management system (QMS) must address.
Throughout this section, you will also observe that various examples are provided showing how a modern electronic QMS will streamline these processes.
NOTE
Please note that the following details are by no means a comprehensive guide to EU MDR QMS requirements. Your medical device company should always follow the official applicable requirements.
1. Regulatory Compliance
Your medical device company’s QMS must have a policy for regulatory compliance.
This means that you will adhere to the laws, regulations, guidelines, and specifications that are relevant to your industry.
For instance, the EU MDR clearly states that intra-ocular lenses come under Class IIb (Medium to High Risk). When your company is marketing these devices in the European market, you have to comply with the guidelines. You cannot market this product as Class IIa (Medium risk) or Class I (Low risk).
2. Conformity of Devices
Your medical device company should ensure that all devices that are in production conform.
According to this regulation, you have to maintain records and documents that show evidence that all products manufactured by your company exactly match the specifications, performance, and safety criteria applicable to that device.
If there are any changes, irrespective of changes in design or the harmonized standards must be attended to in a time-bound manner.
For instance, the intra-ocular lenses that you manufacture are made of silicone. Due to supply issues, you have decided to manufacture them out of acrylic, for which a 3rd party supplier is available. Such a change in the design must be immediately documented and the reasons explained.
By streamlining your quality management processes with modern QMS software with robust change management capabilities like SimplerQMS, you can easily keep track of all changes made to your products, devices, and processes.
Recommended Reading
- Medical Device Document Control: What It Is & How to Simplify It
- What is a Medical Device Technical File and How to Structure It?
3. Management Responsibility
According to EU MDR regulations, the senior management of your company should show commitment towards the development and implementation of an effective QMS in accordance with EU MDR.
What does this mean?
Your senior management must communicate with all employees the importance of the customer and regulatory requirements outlined in the regulations. Not only are you required to establish the company’s quality policy, but you must also establish the objectives, provide resources, and conduct reviews in a timely manner.
SimplerQMS software provides your company with a centralized repository that is a single source of truth for quality data. With this repository, you can manage all types of documents or files that will help you keep an overall check over the company’s QMS.
Moreover, this single source of data can be used for the creation of trending reports, such as CAPAs (open/closed), non-conformances (open/closed), training assignments (on time/overdue), and so on.
All this information will be most useful during your management reviews.
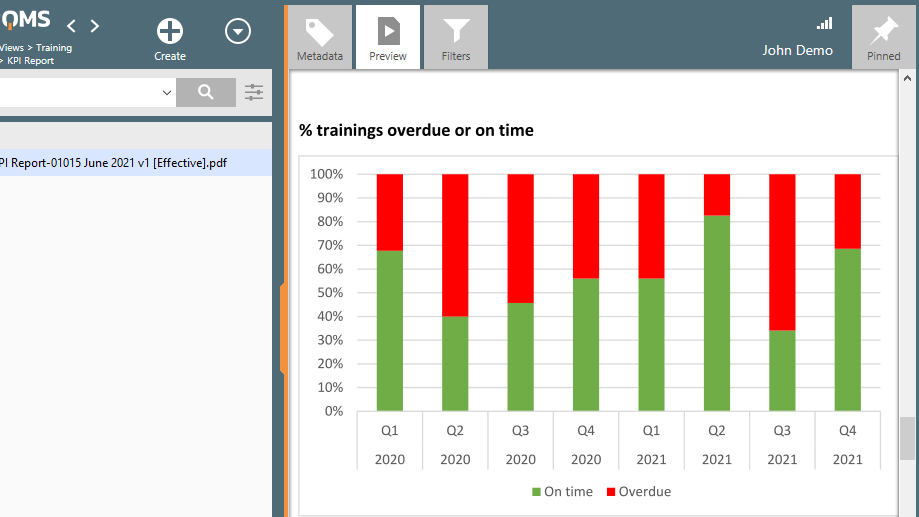
4. Resource Management and Control of Suppliers
Your QMS must include resource management methodologies, including information on selecting and control of suppliers and sub-contractors.
Your QMS must highlight the resources required for the effective implementation of EU MDR. These resources will be both human resources (trained and competent personnel) and infrastructure resources (equipment, space, information systems, and so on).
With the SimplerQMS software solution, you can ensure that all your suppliers are selected following pre-defined criteria. Furthermore, you will be able to make sure that your employees have received the proper training following current SOPs and quality protocols.
Recommended Reading: Medical Device Supplier Management Process (8 Steps)
5. Risk Management
Risk management is an essential component of EU MDR compliance.
All medical devices must be designed, manufactured, and packaged taking into account the purpose the device is intended for.
For instance, the intra-ocular lens we spoke about earlier must be packaged in such a manner that its sterility is maintained. You will not want the lens to inadvertently cause any infection because of a lack of sterility at your end.
A modern QMS software solution should be able to help you accelerate the development of your medical devices, including the processes of verification, validation, and design transfer. All the necessary documentation concerning product realization and risk management would be stored in a centralized repository and easily accessed via highly customizable dashboards.
Recommended Reading: Design Controls for Medical Devices
6. Clinical Evaluation Data and Post-Market Clinical Follow-Up
This is a systematic and planned process by which your company will assess the safety and performance of every medical device that you manufacture and market.
The chief intent of this aspect of EU MDR QMS is to exhibit conformity with the General Safety and Performance Requirements (GSPRs). GSPRs are listed in Annex I of EU MDR and include the intended purpose of the medical device, patient safety, risk control, device properties, and others.
For instance, the intra-ocular lenses that you manufacture are meant to be safe and efficacious for your customers. An unfortunate series of incidents take place wherein these lenses result in adverse events. You are obliged to find out the reasons and ensure that they do not repeat.
With a modern QMS software solution like SimplerQMS, you will be able to continuously collect and monitor data generated from every medical device that is placed on the market. The data may be, for example, in the form of customer complaints, non-conformances, or adverse event reports.
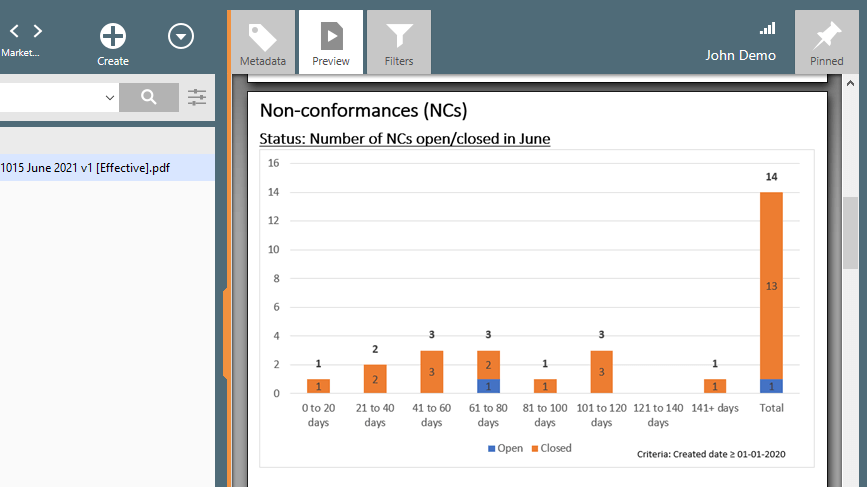
7. Upholding UDI Standards
Your medical device company’s quality management system must be able to make sure that the UDI for all your devices is verifiable, and you should also be able to ensure consistency and validity.
It goes without saying that spurious medical devices will play havoc with the lives of patients. It is also true that such devices are likely to be labeled with the details of a genuine company, which will then get into the crosshairs of regulatory agencies.
How do you safeguard your medical devices?
This is possible with the Unique Device Identification (UDI) for every medical device that you manufacture and market. As mentioned previously, the EU MDR’s UDI system provides exclusive numeric/alphanumeric codes for every authentic medical device in the market. This will help trace each and every medical device.
8. Communication Protocols
How well you communicate with competent authorities, Notified Bodies, customers, and other stakeholders will reflect on your QMS.
For instance, you should be sure of the regulatory needs for every medical device that your company manufactures, and engage a Notified Body as required. When your regulatory affairs department does it efficiently, you will be meeting the requirements and expectations of the Notified Body.
Annex VII of the EU MDR refers to requirements to be met by Notified Bodies.
The NANDO (New Approach Notified and Designated Organizations) database contains the list of notified bodies, their identification number, contact details, and the tasks for which they have been notified, including medical device CE marking.
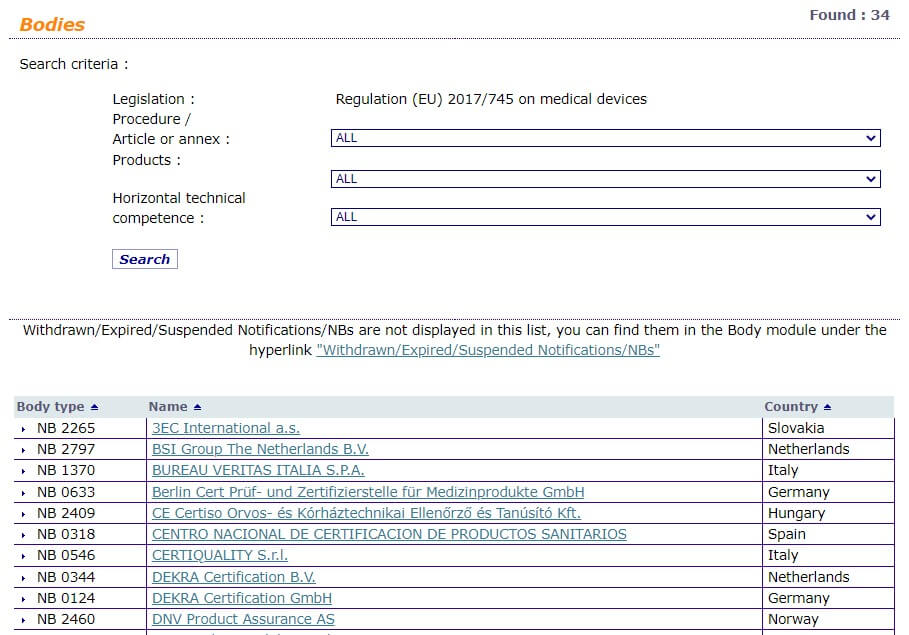
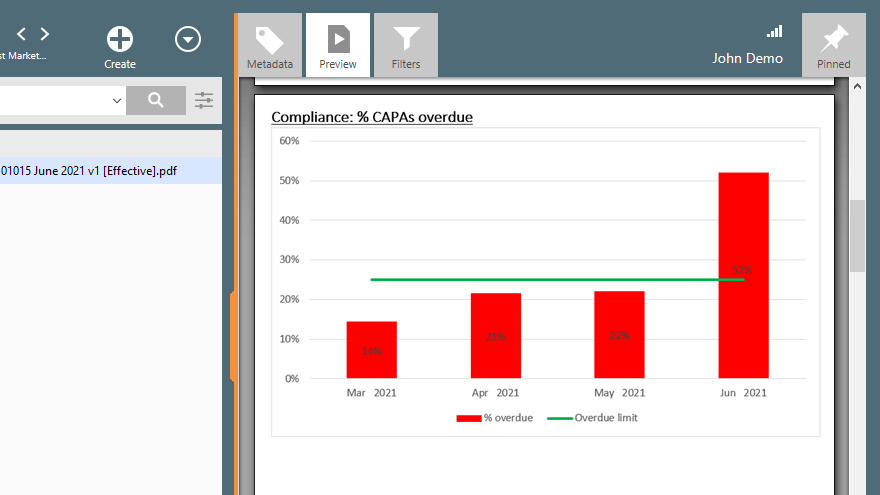
9. CAPA Management
Your QMS system should have processes and protocols in place for managing corrective actions and the verification of their effectiveness.
Let us take for instance the intra-ocular lenses that you market in the European Union. Your Quality Department has received complaints from two locations that the sterility of some of these lenses is compromised. This is a serious issue that must be investigated with full vigor and CAPA generated.
With the CAPA management solution built into QMS software like SimplerQMS, you are assured of more streamlined CAPA management processes. You will be able to recognize and initiate CAPAs, recognize trends, conduct a root cause analysis, initiate actions, as well as verify and close the case with ease.
Recommended Reading: What Is CAPA in the Medical Device Industry?
The Role of QMS Software in Supporting Compliance with EU MDR
When you consider all the EU MDR requirements that were elaborated on in the previous section, you will realize that a traditional paper-based documentation system or a hybrid system will not suffice. They have their own set of challenges, including issues of human error, security, safety, storage, and the burgeoning costs of paper, files, printing, and so on…
Considering the importance of compliance with EU MDR regulations, your company, just like many other medical device companies nowadays should opt for a modern electronic QMS software solution.
With such a quality management solution in place, you will have all the provisions for maintaining compliance with EU MDR, streamlining your operations, increasing efficiency, lowering costs, and being able to confidently market your products in the European Union.
The best QMS software solutions on the market will offer you comprehensive and robust modules for document control, change management, training management, CAPA management, customer complaints, audits, and much more.
Centralized, cloud-based QMS solutions will offer you even more benefits with their anytime, anywhere access, automatic updates, and the ability to integrate with other enterprise software solutions.
Furthermore, features like, automated document routing, document versioning, version control, electronic signatures, time-stamped audit trails, access controls, and permissions will all contribute to the success of your compliance with various regulatory standards and guidelines.
Medical device QMS software by SimplerQMS is an excellent example of such a solution that offers you all the features and benefits that we have elaborated on.
SimplerQMS is a modern, cloud-based quality management software that helps companies in various industries streamline their operations and remain compliant with multiple standards and guidelines, including the EU MDR.
If you would like to assess whether investing in a quality management software solution is the best course of action for your company, then we suggest you download our eQMS Business Case template. It helps quantify the value of investing in quality management software for your business, and present it to decision-makers in your company.
Final Thoughts
Medical device companies are amongst the most highly regulated in the life science industry. This is essential considering that medical devices play such a huge role in the lives of patients.
If your medical device company wants to manufacture and market in the EU, you must be compliant with the EU MDR guidelines which have replaced the EU MDD with effect from 26 May 2021.
When you look specifically at the QMS requirements for medical device companies, you must be thorough with Article 10 (9) of EU MDR.
Traditional paper-based or hybrid QMS systems have no place in modern organizations because of their inherent difficulties and challenges. If you are keen on investing in an eQMS solution to streamline your QMS processes and make compliance with EU MDR easier, book a demo of SimplerQMS and talk to our quality solution experts today.