Change control in the pharmaceutical industry is a systematic approach to managing product, process, or system changes.
It is a critical part of quality management in the pharmaceutical industry, as it helps to ensure that changes are introduced in a controlled manner and that the impact of those changes is fully understood.
This article will discuss change control in the pharmaceutical industry, covering the different types of changes, classification categories, requirements, process flow, and best practices. We will also explore the role of eQMS software in change control management.
SimplerQMS provides eQMS software with comprehensive change control capabilities designed specifically for Life Science companies. You can book a demo to discover how SimplerQMS can support your company’s compliance and quality management efforts.
The following topics will be covered in this article:
- What Is Change Control in the Pharmaceutical Industry?
- What Are the Different Types of Changes?
- What Are the Different Change Classification Categories?
- What Are the Change Control Requirements in the Pharmaceutical Industry?
- What Is the Change Control Process Flow?
- What Are the Recommended Best Practices in the Change Control Process?
- What is the Role of Software in Streamlining Change Control Management?
What Is Change Control in the Pharmaceutical Industry?
Change control in the pharmaceutical industry is a set of controlled actions used to manage modifications to processes, systems, or documents that may impact product quality and safety.
It helps ensure that the modification does not alter the intended process output and adheres to all the quality requirements related to the specific product or process.
The pharmaceutical industry has varying definitions of change control depending on the regulatory requirements and regulatory agencies.
Change control definitions based on some of the distinct guidelines and regulations applicable to pharmaceutical companies are mentioned below.
- EudraLex Volume 4 GMP Annex 15:
The European Union guideline for Good Manufacturing Practices (GMP) defines change control as a structured system where qualified representatives review changes that may impact validated facilities, systems, equipment, or processes, according to EU GMP Annex 15. The goal is to ensure and document that the system’s validated state is maintained. - ICH Q10:
The international guideline for the pharmaceutical quality system model defines the change management process as a systematic approach to proposing, evaluating, approving, implementing, and reviewing changes. - FDA 21 CFR Part 211:
The United States Food and Drug Administration (FDA) defines changes as written procedures that must be drafted, reviewed, and approved by the appropriate quality control team.
What Is the Objective of Change Control?
The objective of the change control process is to ensure that all changes are carefully evaluated, authorized, implemented, and recorded in a controlled manner to reduce potential risks and maintain compliance with regulatory requirements.
Why Is Change Control Needed?
Change control is needed to maintain the uniform and high quality of pharmaceutical products, ensuring they are safe, effective, and compliant with regulatory requirements.
By controlling changes, pharmaceutical companies can mitigate potential risks, document modifications, and ensure that alterations are well-justified. This also reduces disruptions to operations and enables optimal use of available resources.
When Is Change Control Required?
Change control is required whenever proposed or actual modifications or alterations are made to processes, systems, equipment, facilities, materials, or documents that could impact the quality, safety, or efficacy of products in the pharmaceutical industry.
Having a robust change control process allows pharmaceutical companies to manage different types of changes.
What Are the Different Types of Changes?
Changes are broadly categorized into two main types: planned and unplanned.
Unplanned Changes
An unplanned change is a modification that occurs unexpectedly and requires immediate attention.
These often result from unforeseen events, such as equipment failures, safety incidents, customer complaints, deviations from established procedures, and other events.
Deviations are considered unplanned changes, and it is important to have a process in place for properly managing deviations that impact product safety, efficacy, and quality.
Planned Changes
A planned change is an intentional and pre-approved modification implemented after a detailed evaluation and authorization through the change control process.
These changes are carefully considered, and their impact on product quality, safety, and compliance is assessed before implementation.
Change types are broad categories of modifications. A more detailed approach is the change classifications based on their impact and significance on the resulting product, process, or system.
What Are the Different Change Classification Categories?
Changes can be classified into three categories based on their impact:
- Minor changes
- Major changes
- Critical changes
Minor Changes
Minor changes are modifications with minimal impact on the resulting product, process, or system.
Minor changes usually involve minor adjustments or improvements that do not significantly affect product quality or safety. For example, changing the font size on product labels.
Major Changes
Major changes have a more substantial impact on the resulting product, process, or system.
Major changes may require careful evaluation and validation to ensure a uniform and high level of product quality. For instance, modifying the manufacturing process for a drug formulation.
Critical Changes
Critical changes are modifications that have a significant potential effect on product quality, safety, or efficacy.
Critical changes may demand rigorous assessment, validation, and regulatory approval. An example would be changing the active ingredient in a pharmaceutical product.
What Are the Change Control Requirements in the Pharmaceutical Industry?
Change control requirements in the pharmaceutical industry vary depending on the market companies operate and the applicable requirements.
Some requirements for the change control process include the following.
NOTE
The following guidelines are applicable to change control management in the pharmaceutical industry. However, this is not an exhaustive list. Please always refer to the official requirements applicable to your company.
EudraLex Volume 4 GMP
The EudraLex Volume 4 GMP offers guidance for manufacturers of human and veterinary medicinal products within the EU on interpreting the GMP principles. As part of Good Documentation Practices, Chapter 4, Section 4.29 states that there must be written procedures and protocols for the change control process.
In Chapter 5, Section 5.25 outlines that any change in the manufacturing process, equipment, or materials, which may affect product quality and process reproducibility, should be validated.
Additionally, within Annex 15 Section 11, the qualification and validation guidelines provide specific details and instructions regarding change control. It emphasizes that quality risk management should be employed to assess planned changes and anticipate their effects on product quality, avoiding unintended consequences.
EU 1252/2014
The European regulation EU 1252/2014 specifies the GMP requirements that manufacturers and distributors of active substances for medicinal products must comply with.
The requirements for change control are outlined in Article 14. The requirements specify that companies must assess how changes to the manufacturing process may affect the quality of the active substance before implementing them.
FDA 21 CFR Part 211
The FDA regulation 21 CFR Part 211 establishes the current Good Manufacturing Practice (cGMP) requirements for finished pharmaceutical products.
The change control process is mentioned in the following sections:
- 21 CFR 211.22: Authorizes the Quality Control Unit to approve or disapprove changes. The company is responsible for providing quality control personnel with all necessary resources and facilities.
- 21 CFR 211.100: Describes the requirements of written procedures for production and process control, including changes. These procedures must be written by authorized personnel and approved by the Quality Control Unit. In the event of a deviation from written procedures, it must be recorded and justified.
- 21 CFR 211.160: Outlines the requirements of written procedures in a laboratory environment, covering various processes, including changes.
Our dedicated article provides a comprehensive explanation of cGMP, including its importance, requirements, and how to maintain compliance.
ICH Q10
The ICH Q10 is a guideline that specifies a model for an effective pharmaceutical quality management system.
Section 3.2.3 states that the change control process should provide a high level of confidence that no unintended consequences result from the change. Quality risk management should be used to assess proposed changes.
Pharmaceutical companies can utilize specific steps within the change control process to meet established requirements and systematically evaluate, authorize, document, and implement changes.
You can learn more about this guideline in our dedicated article about the ICH Q10 Pharmaceutical Quality System.
What Is the Change Control Process Flow?
The steps of change control process flow involve specific actions for managing changes to a product, process, or system.
The steps involved in the change control process will vary depending on the company and the change classification category.
We will discuss change control process steps and provide examples of how an electronic QMS (eQMS), like SimplerQMS, further streamlines each step of the change control process.
Common change control process flow steps are listed below:
- Initiate Change Request
- Perform Impact Assessment
- Review Change Request
- Approve Change Request Plan
- Implement Change
- Provide Training (As Applicable)
- Monitor Change Effectiveness
1. Initiate Change Request
The first step of the change control process involves filling out a change request form or following a defined change control procedure to identify and formally document the proposed change.
Company criteria and internal procedures establish the necessity for change and can be based on change classification, such as minor, major, and critical, to implement alterations.
For example, minor changes may not require significant process alterations. In contrast, major and critical changes demand more rigorous evaluation before implementation.
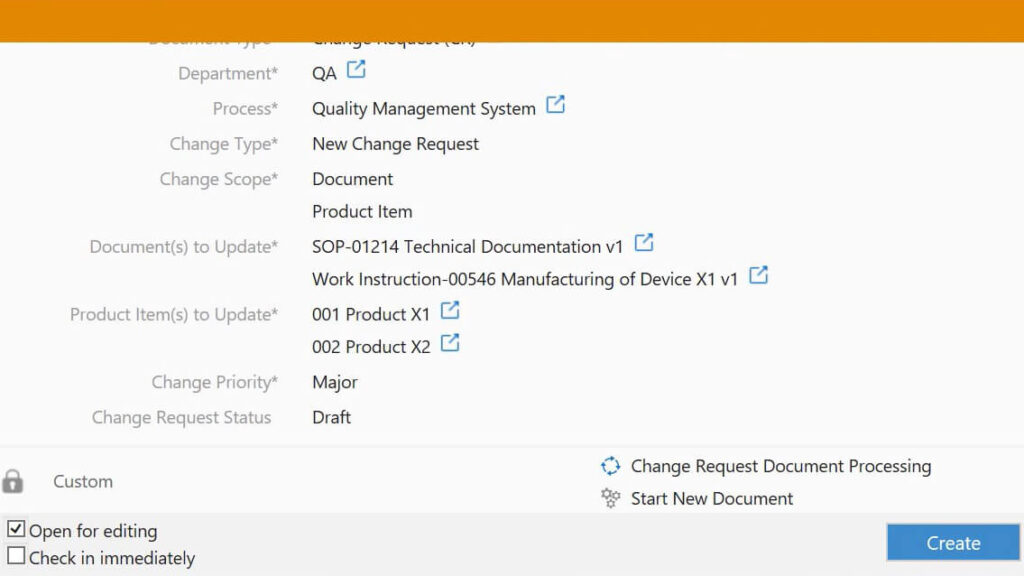
SimplerQMS provides an eQMS software solution with a comprehensive change control management module.
Companies can easily classify changes when drafting change request documents by selecting the type and priority of change from the dropdown menus.
2. Perform Impact Assessment
Once the change request is initiated, an impact assessment may be conducted. This step involves evaluating the proposed change’s potential impact on product quality, safety, efficacy, and regulatory compliance.
Quality risk assessments may also be performed to identify potential risks associated with the change.
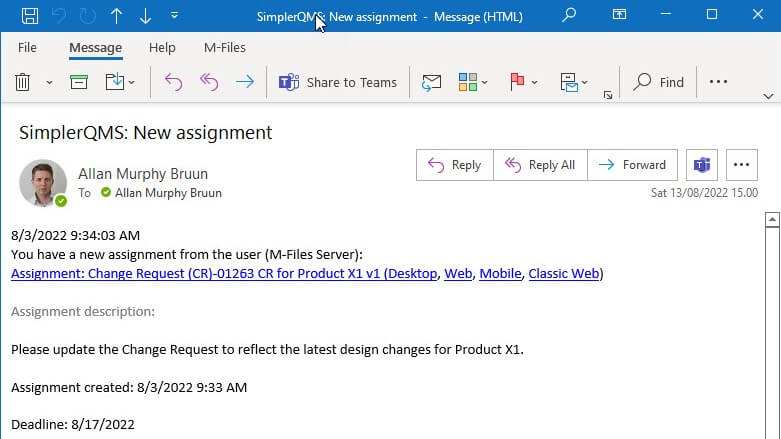
SimplerQMS helps ensure quality risk assessment tasks or any other tasks are performed on time. The system automatically sends reminders and notifications to the assigned people about activities’ required actions and due dates.
3. Review Change Request
The change request is reviewed by assigned personnel with the relevant qualifications.
All relevant information and data are examined during this stage.
SimplerQMS provides pre-defined workflows to guide users through the steps in the change request process flow. Routing documents for review and approval to the assigned persons is done with just a few clicks.
4. Approve Change Request Plan
After the impact assessment and change request review, the responsible person approves or rejects the change request plan.
Major or critical changes may require approvals from multiple people.
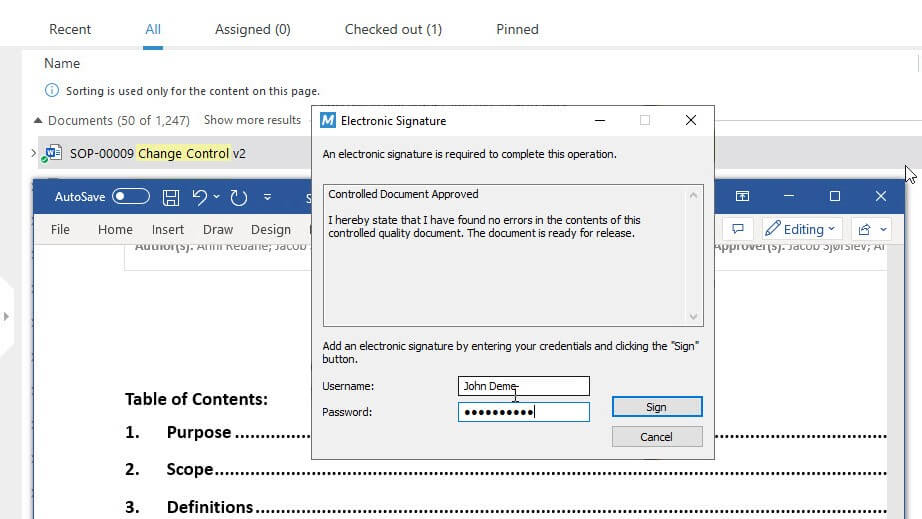
In SimplerQMS, change request plans are approved and signed off using 21 CFR Part 11 compliant electronic signatures for secure signing of documents. Users sign documents using unique identification codes and password combinations, ensuring accountability and traceability of all actions.
5. Implement Change
After approval and training, the approved plan of change is implemented in a controlled and systematic manner.
Implementing a change includes verifying that the necessary adjustments are made to processes, equipment, or documentation as required. Afterward, the change request is approved for closure.
6. Provide Training (As Applicable)
After the change control process, it is possible to provide training to all relevant personnel on the approved changes, if necessary.
Training ensures that everyone involved understands the new processes, procedures, and modifications related to the change. Adequate training is essential for a smooth and successful implementation of the change.
Effective communication ensures that everyone is aware of the change, its purpose, and its impact on their roles and responsibilities.
SimplerQMS offers robust training management capabilities, helping streamline training processes within the company.
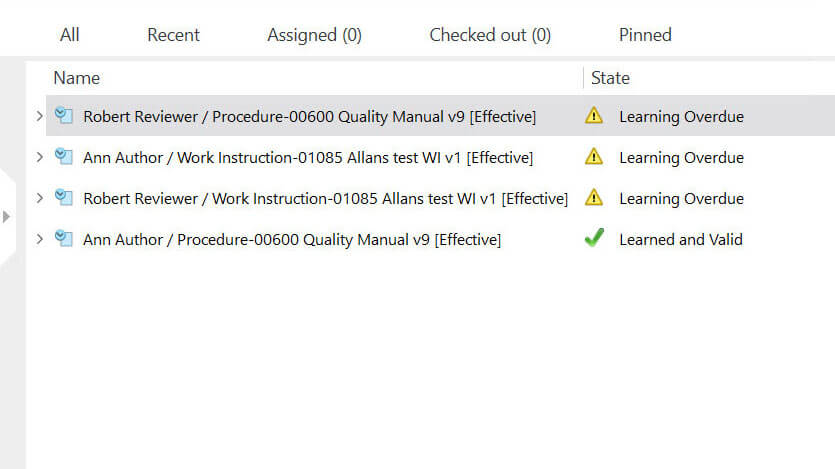
For example, Training Managers can assign specific employees to relevant training groups and create learning rules. Employees in specific training groups can get automated notifications regarding retraining when related documents, such as SOPs (Standard Operating Procedures), are updated.
Each employee’s training assignment’s progress can be monitored via highly customizable views.
7. Monitor Change Effectiveness
Once the change is implemented, its performance is monitored and evaluated. This involves conducting an effectiveness check to evaluate the impact and success of the implemented change.
The monitoring process helps identify potential issues and ensures that the change delivers the desired results. During this phase, any deviations from expected results are identified and addressed.
With SimplerQMS, you can assign specific tasks to individuals responsible for assessing the effectiveness of changes. These tasks involve periodic reviews to ensure that the changes are working as intended.
The system can automatically send reminders and notifications regarding assessment due dates, helping to ensure effectiveness checks are carried out in a timely manner.
What Are the Recommended Best Practices in the Change Control Process?
Following best practices for change control processes helps pharmaceutical companies ensure that their change control process is effective and that changes are made in a safe and controlled manner.
Some of the recommended best practices for the change control process are listed below:
- Have a well-defined change control process: An organized and documented change control process that outlines the steps, responsibilities, and procedures for managing changes should be established. This ensures consistency and clarity throughout the change process.
- Keep clear change request documentation: All requests need to be thoroughly documented, including the reason for the change, its scope, potential impact, and proposed solutions. Proper documentation facilitates a comprehensive evaluation and decision-making process.
- Conduct risk assessments: A risk assessment for each proposed change must be performed to identify potential risks and their impact on product quality, safety, efficacy, and regulatory compliance.
- Perform testing and validation of changes: Changes with a high impact on product quality, safety, or efficacy should be tested and validated before implementation. These activities help ensure the change is well-controlled and does not introduce new risks or potential issues.
- Maintain comprehensive documentation and record-keeping: Documentation and record-keeping must be organized throughout the change control process. This includes change request forms, risk assessment reports, approval records, implementation plans, and effectiveness check records.
Following these best practices can help pharmaceutical companies ensure that their change control process is effective. Additionally, QMS software solutions help to automate and streamline the change control process, making it even more efficient and effective.
What is the Role of Software in Streamlining Change Control Management?
Traditionally, the change control process involved manual paper-based and hybrid systems, leading to excessive paperwork, potential for human error, and time-consuming management.
A more efficient solution is adopting an electronic Quality Management System (eQMS) offering built-in support for change control management. An eQMS software integrates all quality processes and centralizes all information in one system.
SimplerQMS provides eQMS software with comprehensive change control management capabilities. Our system is tailored to Life Science companies’ needs.
With our change control management software module, pharmaceutical companies can streamline change control processes from change request submission to final approval and successful implementation.
You can create change requests directly from a customer complaint and have a seamless workflow. Automated workflows facilitate routing change requests for review or approval and assigning specific people as responsible persons. The system automatically sends reminders and notifications to ensure the change request is handled on time.
SimplerQMS prevents unauthorized changes in documentation and processes by limiting access to the system. This way, change requests, and other specific documents can only be accessed by authorized personnel.
Documents in our eQMS are approved using electronic signatures. We offer 21 CFR Part 11 compliant electronic signatures software for secure and effortless document sign-off.
In addition to 21 CFR Part 11, our platform supports compliance with several Life Science requirements such as ISO 9001:2015, ISO 13485:2016, FDA 21 CFR Part 210, 211, and 820, EU GMP, EU Annex 11, and more. By providing comprehensive QMS process support, SimplerQMS software helps companies achieve regulatory compliance.
SimplerQMS solution supports QMS processes, including document management, change control, employee training, deviation management, CAPA management, customer complaint management, audit management, supplier management, and more.
If you are unsure about the benefits of eQMS implementation, we recommend downloading our eQMS Business Case template. It offers a structured approach to assess the value of eQMS for your company, including factors like cost savings, improved efficiency, and enhanced regulatory compliance.
Present a compelling case to your management or board for adopting an eQMS solution using this template.
Final Thoughts
Change control is an essential part of quality management in the pharmaceutical industry.
By following a well-defined change control process, companies can ensure that changes to products, processes, and systems are made in a safe and controlled manner. This helps to ensure product quality, safety, and compliance with regulatory requirements.
To manage change control, companies traditionally used paper-based and hybrid systems. However, these systems are susceptible to errors, and document loss, and require physical space for storage.
As a solution to these challenges, pharmaceutical companies are increasingly embracing modern eQMS software solutions to streamline change control management.
SimplerQMS provides eQMS software with comprehensive change control and quality management capabilities specifically designed for Life Science companies.
You can schedule a free demo with one of our quality consultants to understand how our QMS software can help you streamline quality process management in your company.