QMS Software for Pharmaceutical and Biotechnology Industries
Optimize workflows and reduce human error by streamlining your quality management processes.
TRUSTED BY
























Streamline Processes and Achieve Compliance With a Pharmaceutical QMS Software
Pharmaceutical and biotechnological industries are highly regulated due to their impact on patients’ lives. Companies must comply with several requirements related to document management, change control, training management, and other pharmaceutical QMS processes.
SimplerQMS provides complete QMS software to help you work more efficiently and ensure compliance with these requirements. Our solution offers all the relevant QMS modules and is fully validated and pre-configured based on Life Science requirements.
Maximize Efficiency With an Interlinked eQMS
Have all QMS modules integrated into one pharmaceutical quality management software.
Easily manage your procedures, as well as forms and templates across all your quality management processes. Or utilize our complementary procedure and template package, which meets the exact requirements of most life science companies.
Link related documents across different processes, from document management and training to suppliers to audits, deviation handling, and other processes.
Seamless Integration With Microsoft Office Apps
Integrate familiar Microsoft Office applications, such as Word, Excel, PowerPoint, and Outlook, with SimplerQMS software and keep working with well-known tools.
Edit and save documents in SimplerQMS Cloud with just a few clicks. No need to download and upload files manually.
Streamline Processes With Automation
Automate data collection, reminders, notifications, periodic tasks, and assignments.
The system automatically records all actions and creates a complete time-stamped audit trail.
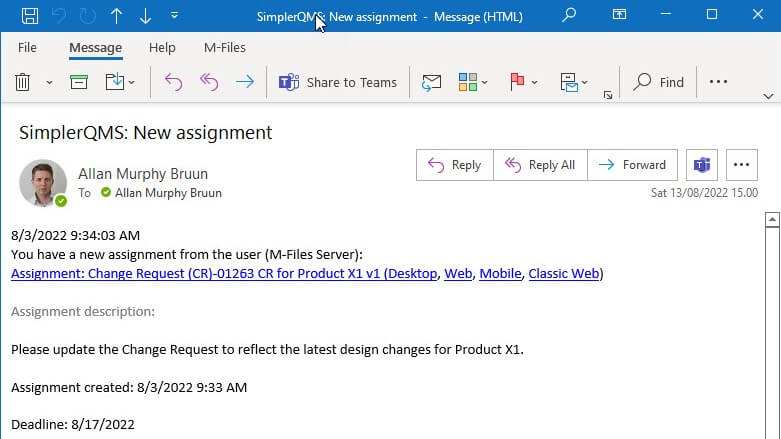
Create assignments and define timelines to get automatically notified of required actions before the due dates.
Ensure ongoing learning in case documents are updated with automatic email notifications being sent to relevant personnel regarding retraining.
Ensure Compliance With Life Science Requirements
SimplerQMS supports companies in achieving compliance with requirements in the Life Science industry, such as GxP guidelines and regulations, ICH guidelines, FDA regulations, ISO standards, and many others.
We offer a complete QMS software solution with state-of-the-art document management capabilities, FDA 21 CFR Part 11, and EU Annex 11 compliance.
Easily create documents using our complementary template package and streamline processes through our pre-configured workflows.
SimplerQMS is also ready to participate whenever your company is audited or inspected, and processes need to be addressed regarding the eQMS.
Maintain Document Control With Robust Features
Ensure easy access to documents and maintain document integrity with our robust features.
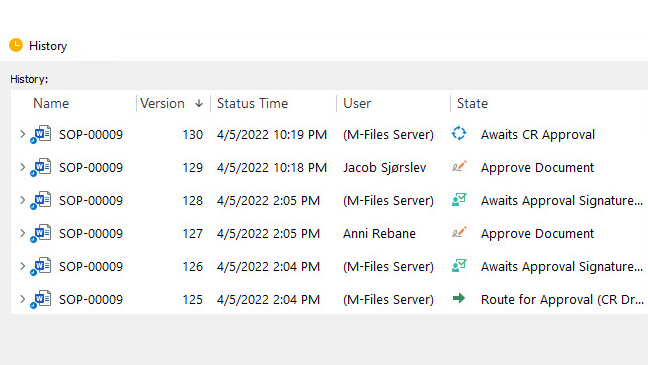
SimplerQMS supports the most sophisticated document management processes, including document version control and document approvals.
Search for documents using keywords in the title and content to facilitate document retrieval.
Receive automatic reminders and notifications of tasks before due dates to ensure work is completed on time.
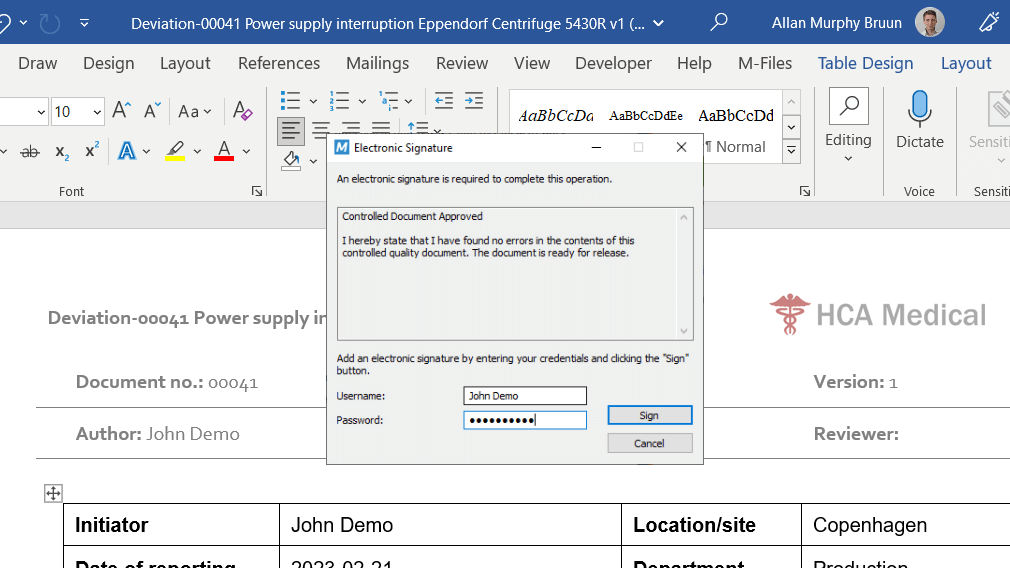
Electronically sign documents with FDA 21 CFR Part 11 and EU Annex 11 compliant electronic signatures.
Easily Control and Manage Changes
Streamline change control processes, including change requests and approvals.
Use a pre-configured workflow to address all steps in change requests, including creating documents using templates, routing changes for review and approval, and assigning tasks to specific people.
Electronically sign the change request upon approval to document the change justification and automatically record all steps along the way.
Check the effectiveness of a specific change by setting reminders for periodic reviews and ensure necessary actions are taken on time.
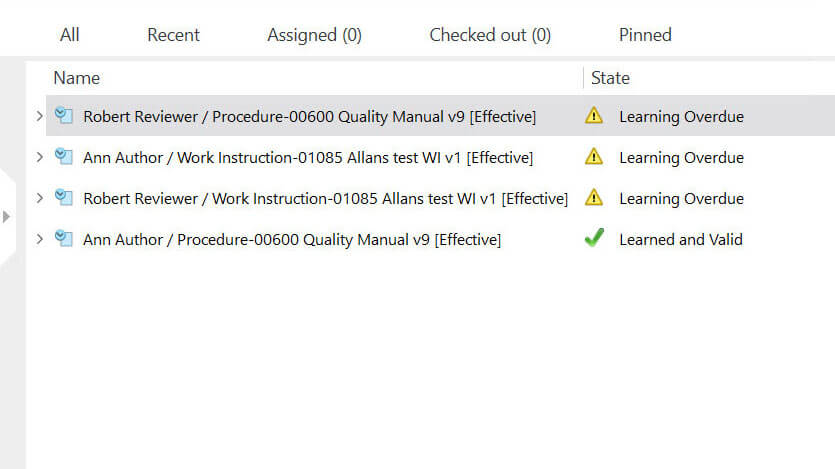
Simplify Employee Training Management
Ensure your employees are always up to date on required training and everything is documented properly.
Establish comprehensive training plans and assign specific roles to employees.
Automate notifications to employees when training due dates are approaching or when retraining or review is necessary.
Monitor each employee’s training progress and create reports on training completion.
Manage Assessments of Training Effectiveness
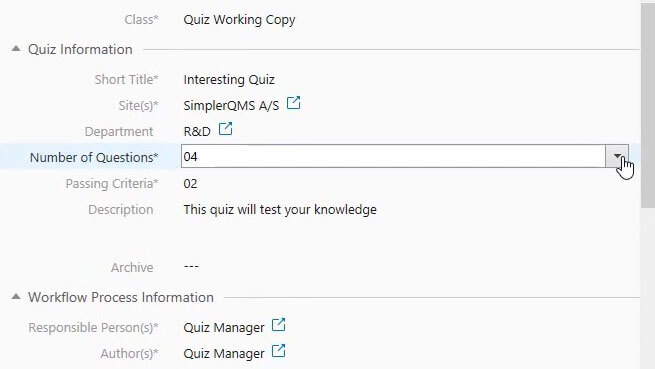
Measure training effectiveness with customizable quizzes in multiple-choice format and pre-defined passing criteria.
Easily link quizzes to learning documents. Send the quiz through the “draft, review, and approve” workflow before releasing it and route it for updates when needed.
Go beyond simply having trainees read and sign documents – evaluate training effectiveness more accurately with quizzes.
Efficiently Manage and Resolve Deviations
SimplerQMS simplifies the process of managing and resolving deviations.
Create a deviation document to address undesired events or differences from specified requirements. Assign specific roles to relevant persons and route deviations for review and approval.
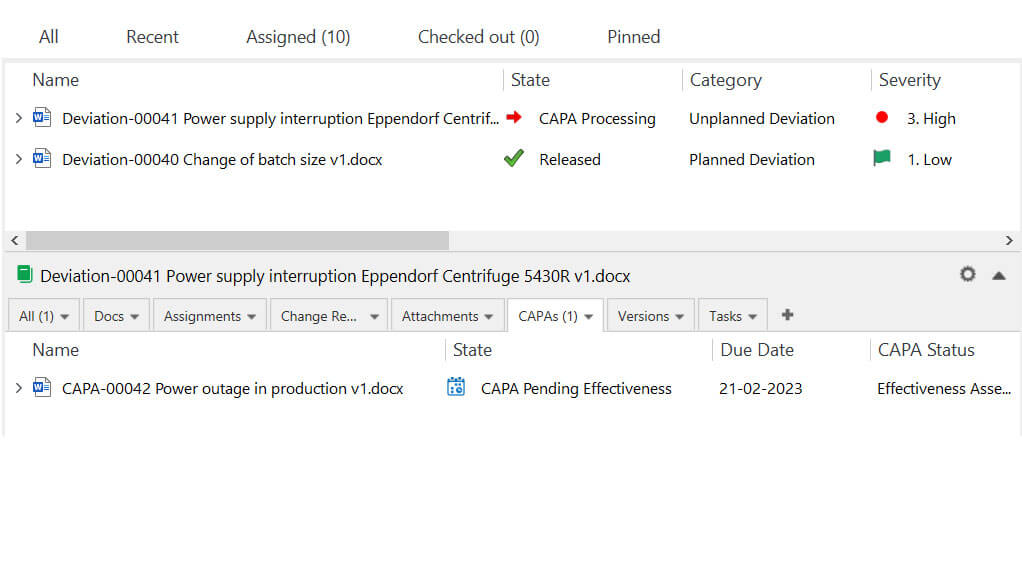
Escalate deviation to the CAPA process with just a few clicks and have all information interlinked. Schedule checks to assess the effectiveness of actions.
Monitor the progress of each deviation and create reports for trend analysis of deviations.
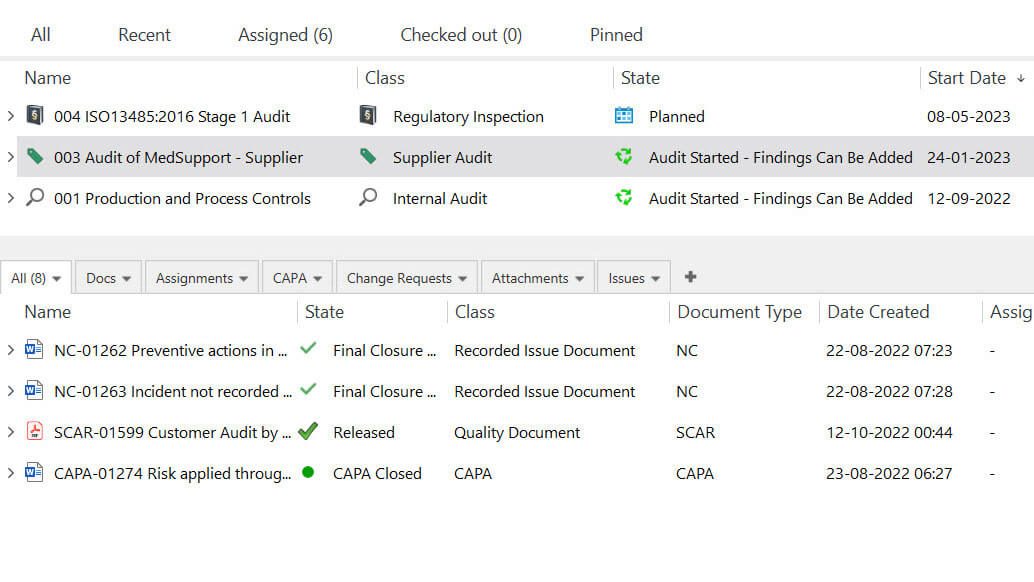
Proactively Address Issues With CAPA Management
Create new CAPA documents using pre-defined templates to ensure accuracy and consistency in the process. Create as many CAPAs as necessary, depending on the severity of the problem.
Route CAPA documents for review and approval, assign tasks to responsible persons and automate notifications of required actions before due dates.
Easily link a CAPA to other related documents such as deviation, complaints, and audit findings.
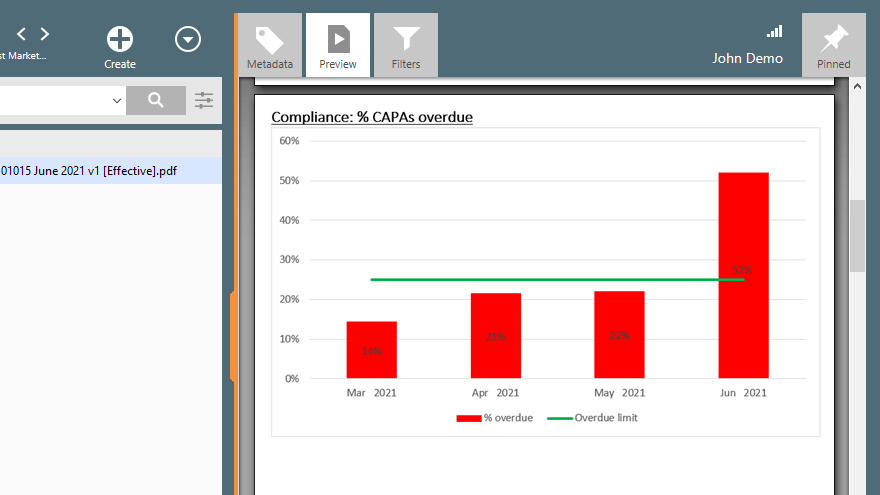
Monitor the status of each CAPA with highly customizable views. Generate CAPA reports to track progress, such as the percentage of overdue CAPAs, compare it to pre-set overdue limit, and more.
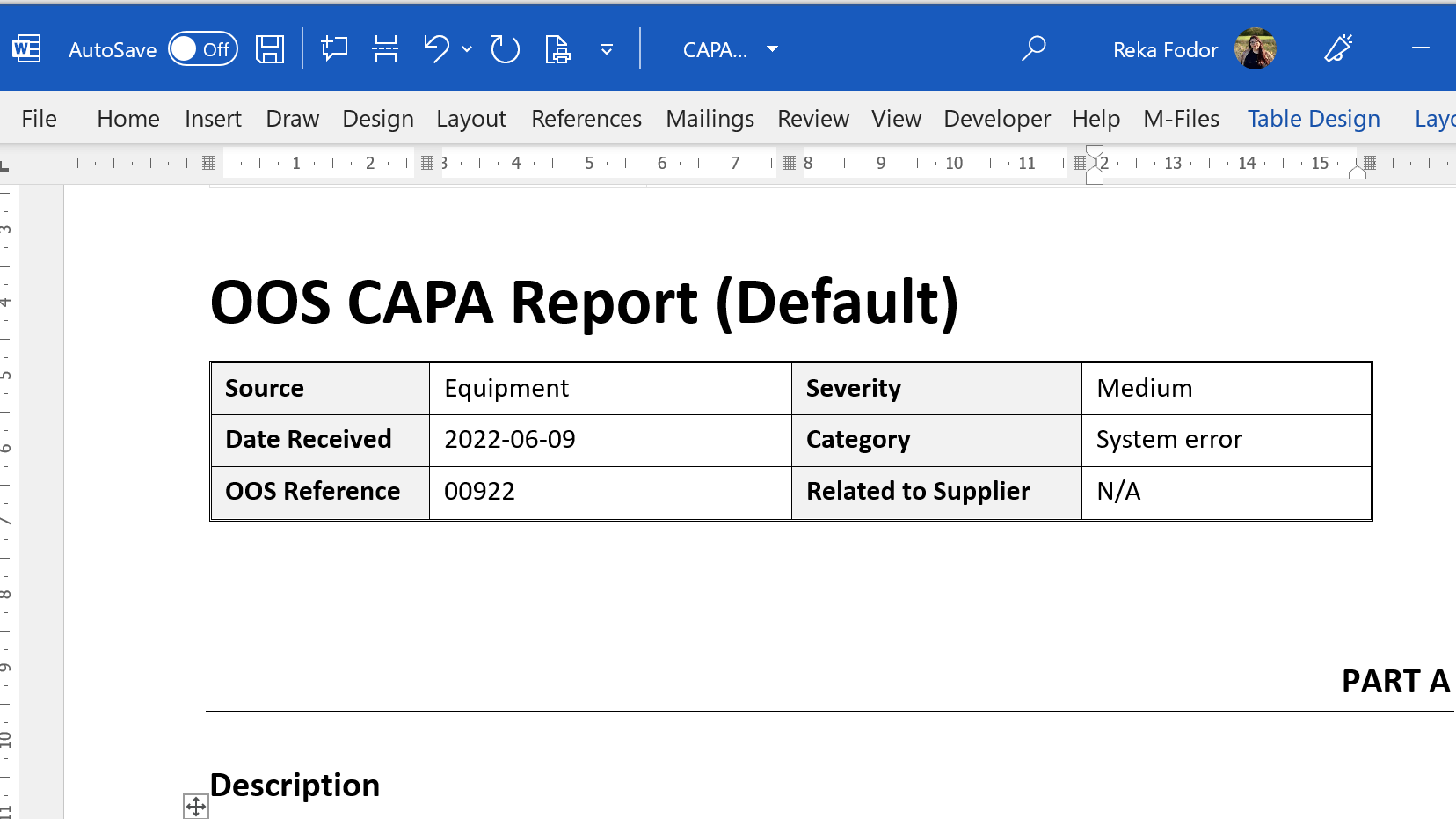
Stay On-Track With Out-of-Specification and Out-of-Trend Result Handling
Create Out-of-Specification (OOS) and Out-of-Trend (OOT) investigation documents based on your company’s standards.
Link OOS and OOT documents to related documents, such as processes, products, departments, equipment, and raw materials, to ensure traceability. Automate notifications and reminders of upcoming due dates for investigation, review tasks, and required actions.
Generate reports based on OOS and OOT results to identify trends and take preventive actions. Easily monitor all tasks related to the investigations and their overall statuses.
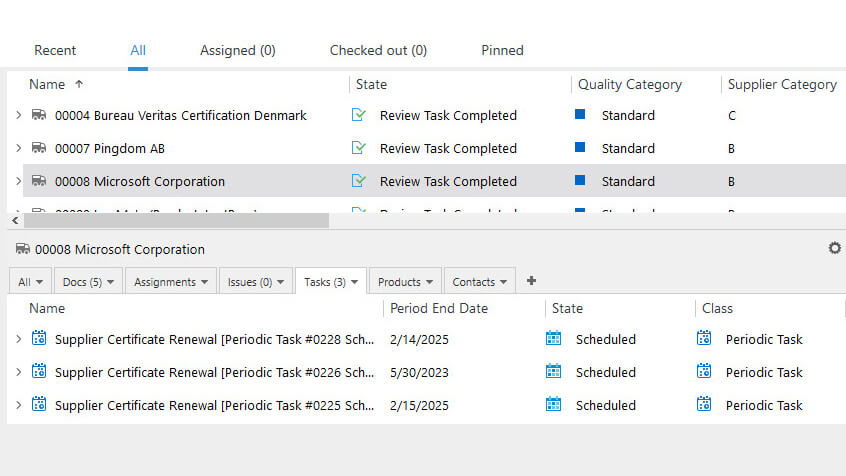
Streamline Vendor Management Processes
Manage vendor qualification, selection, and monitoring processes with ease using pre-configured workflows based on Life Science requirements.
Schedule supplier requalifications, reviews, assignments, and periodic tasks and have an overview of all processes.
Maintain Approved Supplier Lists (ASLs), and manage contracts, certificates, surveys, evaluations, certificates, incoming inspections, and more in one system.
Define access levels to relevant vendor documents to ensure compliance and data security.
Improve Audit Outcomes With Audit Management Module
Create audit plans, then create and link individual audits, such as regulatory inspections, supplier audits, and internal audits, to the audit plan and keep track of all scheduled audit-related activities.
Assign Issue Handlers and enable them to escalate audit findings to CAPAs with just a few clicks and have documents interlinked to ensure full traceability of information.
Search and retrieve documents quickly to answer any audit-related questions during audits and inspections.
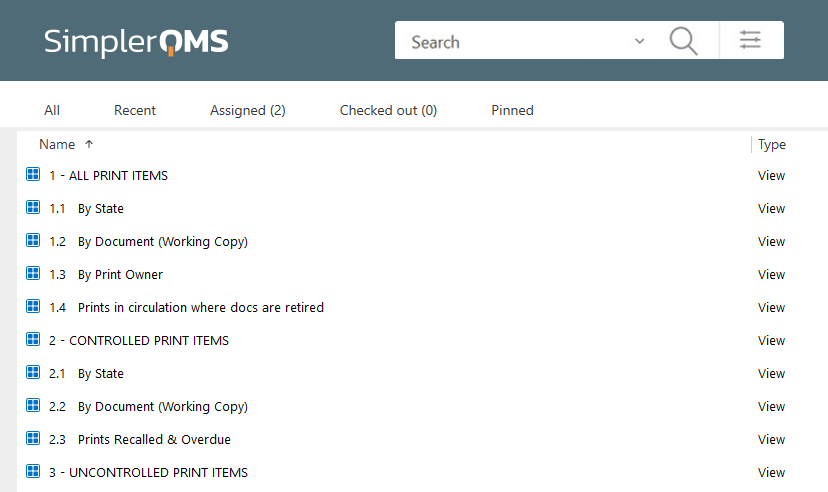
Keep Track of Printed Documents with Controlled Printing
Controlled Printing capability allows users to securely and efficiently print documents, as well as track, manage, and recall printed document copies.
This feature provides information such as unique ID, date, time, print type, and responsible person in the footer to all printed documents, with a “COPY” overlay for uncontrolled prints, allowing users to quickly and easily identify different copies.
It also allows for the recall of documents to ensure outdated materials are destroyed.
Transparent Pricing for Maximum Budget Control
All SimplerQMS modules and features are included in one subscription price. The total price varies depending on the number of licenses you acquire.
So, there are NO extra fees for implementation, hosting, system validation, user training, ongoing support, or additional modules. Everything is covered. It is that simple.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Complete eQMS for Pharma and Biotech Companies
Training Management
Design and implement comprehensive training plans, automate notifications, and monitor progress.
CAPA Management
Identify, uncover, resolve, and report all the preventive and corrective actions seamlessly.
Complaint Management
Record customer complaints, track resolutions, and analyze trends for continuous improvement.
Change Control
Manage all changes accordingly to ensure structure and compliance within your company’s QMS.
Audit Management
Streamline audit management processes and reduce the time and effort needed to pass audits successfully.
Document Management
Simplify all document creation, approval, versioning, and storage processes in one system.
Risk Management
Identify, analyze, and reduce risks within your company through effective risk management.
Vendor Management
Simplify vendor-related activities and manage vendors according to requirements.
Equipment Asset Management
Easily control and manage equipment, equipment maintenance, and calibration tasks.
Frequently Asked Questions
What Is a Pharmaceutical QMS Software?
Pharmaceutical quality management system software is a solution that supports pharmaceutical companies in managing their Quality Management System (QMS) processes more efficiently while maintaining compliance.
It includes tools and features necessary to streamline and automate aspects of document management, change control, CAPA management, training management, customer complaint management, audit management, and other quality processes.
Among these features, we can highlight the capability to relate documents to processes, products, customers, vendors, regulatory requirements chapters, and other elements to keep track of all documentation.
What Are the Benefits of Using an eQMS for Pharma and Biotech Companies?
The main benefits of using an eQMS for biotech and pharma companies include improved visibility into their QMS processes, improved efficiency in regard to managing quality-related processes, reduced audit time and effort, improved compliance with Life Science requirements, and reduced costs associated with manual processes.
Additionally, an eQMS can enable better communication between departments, improved collaboration, and better decision-making resulting from improved data visibility.
Is SimplerQMS FDA 21 CFR Part 11 and EU GMP Annex 11 Compliant?
SimplerQMS solution is compliant with 21 CFR Part 11 and EU GMP Annex 11 regarding electronic records and electronic signatures.
How Much Does SimplerQMS Pharma QMS Software Cost?
The cost of the SimplerQMS depends on the type and number of licenses you acquire.
SimplerQMS includes all software modules, hosting, validation, implementation, user training, and ongoing support for one subscription price, so there are no additional fees.
The price is based on the number of licenses you acquire. Please visit our pricing page to learn more about SimplerQMS and get a custom quote.
How Much Time Does It Take to Implement SimplerQMS?
The SimplerQMS solution usually takes five to six weeks to be implemented.
The exact time frame will depend on the size and complexity of your organization. The number of documents to be migrated into the system and available resources also need to be considered.
Our team of experts will work closely with you throughout the implementation process and beyond to ensure a smooth and successful transition to a digital quality management solution.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To learn how you can make the most of SimplerQMS in your life science company – book a tailored demo.