Controlled Printing for the Life Science Industry
Print or download controlled copies of electronically stored documents and keep track of all printouts.
TRUSTED BY
























Controlled Printing Capability Within Complete eQMS
SimplerQMS controlled printing feature allows Life Science companies to manage the printing of documents more efficiently while staying in compliance with the FDA 21 CFR Part 11, Part 211, and EU GMP Annex 11.
Record the printed item’s details, track printout status, and monitor all controlled copies in circulation. Let the system automatically recall prints when a new version is released or issue a recall manually.
Controlled printing is just one feature of a complete eQMS software by SimplerQMS. We provide all Life Science QMS modules such as document control, change management, training management, complaints management, CAPA management, audit management, and much more.
Perform Controlled and Uncontrolled Printing of Documents
Select which document types the controlled printing capability should be enabled for.
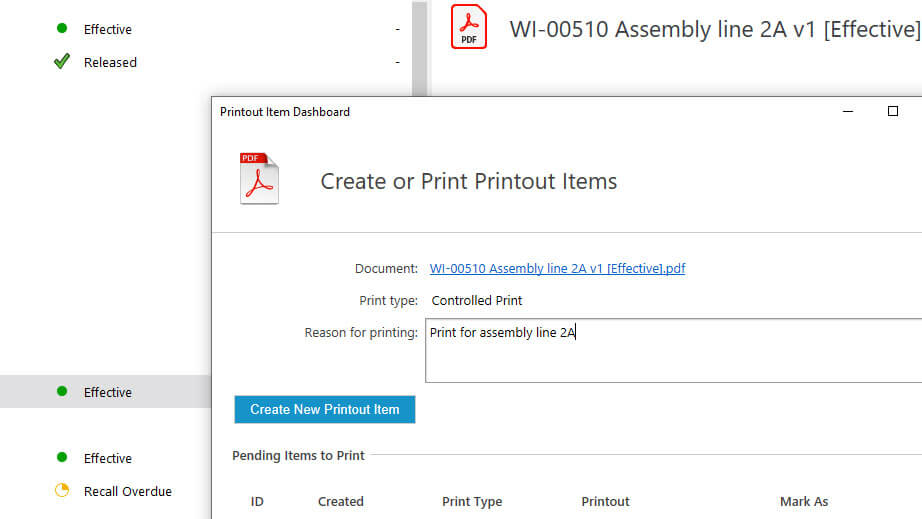
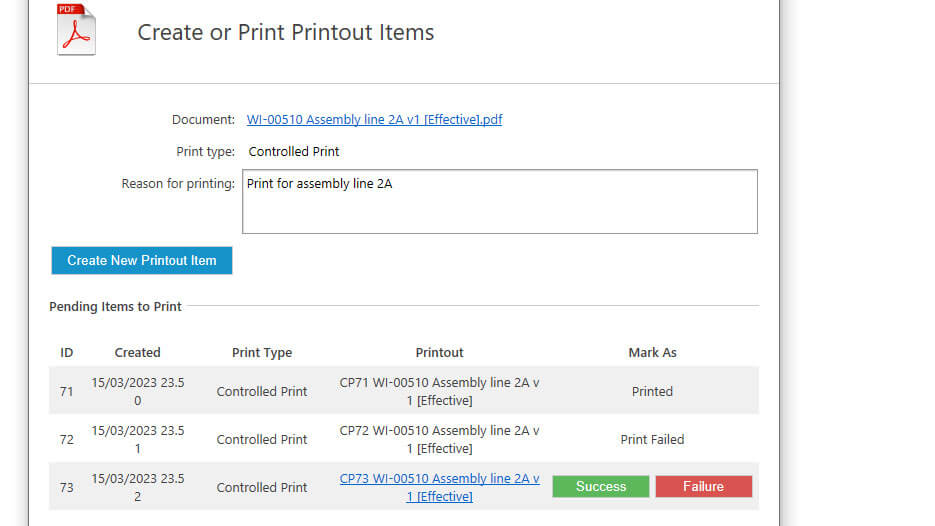
Easily create controlled or uncontrolled printouts of documents by choosing the print type. This determines the unique footer that is added to each page of the printout.
Enter a reason for printing a controlled document to keep full traceability of actions.
Track Who Printed or Downloaded Which Document and When
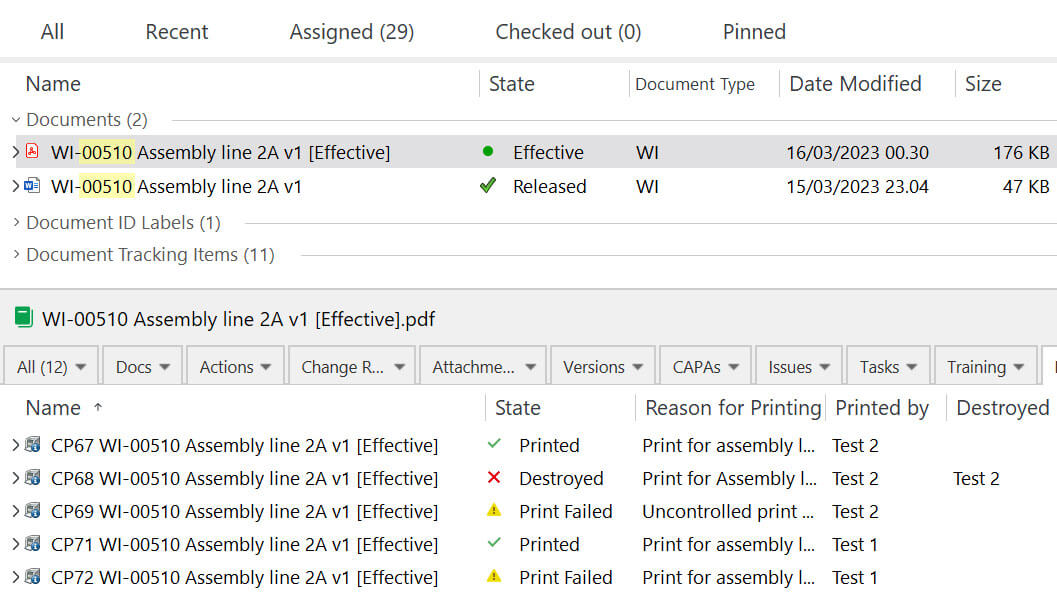
The system automatically records who printed the document.
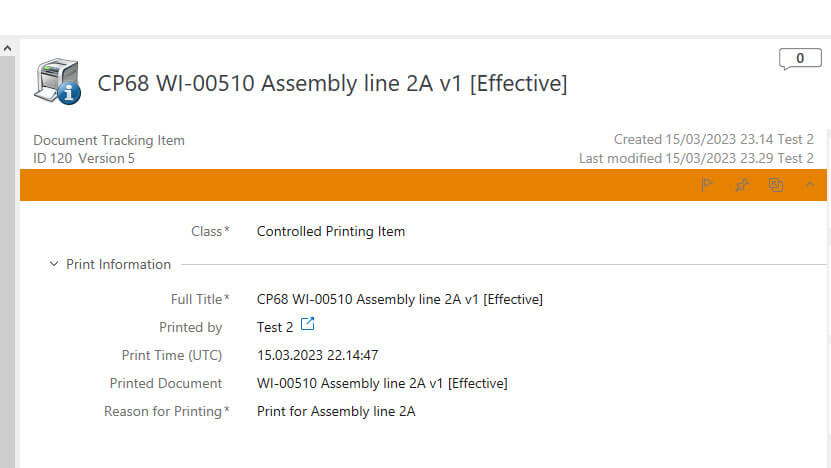
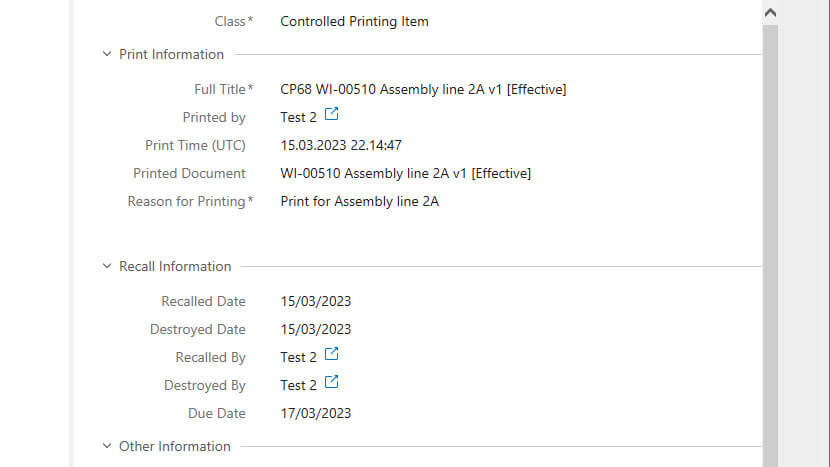
It also records information such as the date, time, print type, reason for printing, and the printing outcome to provide a time-stamped audit trail.
A footer is added on every page of controlled and uncontrolled printouts that includes a unique ID, creation date and time, the person responsible for the printing, and the print type.
Ensure Compliance With Life Science Requirements
Ensure compliance with requirements regarding control of records present in the FDA 21 CFR Part 11, Part 211, and EU GMP Annex 11 by enabling controlled printing for documents. Easily access the history of any document and overview a time-stamped audit trail.
Although not all documents require controlled printing. Therefore, the scope of what documents are controlled is determined for each document type. Additionally, users can select the print type – controlled or uncontrolled for document types in scope.
Uncontrolled copies can be used to provide access to a document that may not be the latest version and will be clearly marked as uncontrolled.
Quickly Recognize Different Document Copies
Create controlled printouts with a unique ID, date, creation time, print type, and the responsible person’s name in the footer on all document pages.
Identify uncontrolled printouts with a “COPY” overlay in the header of each page, as well as the document footer information.
Recall and Destroy Controlled Print Items
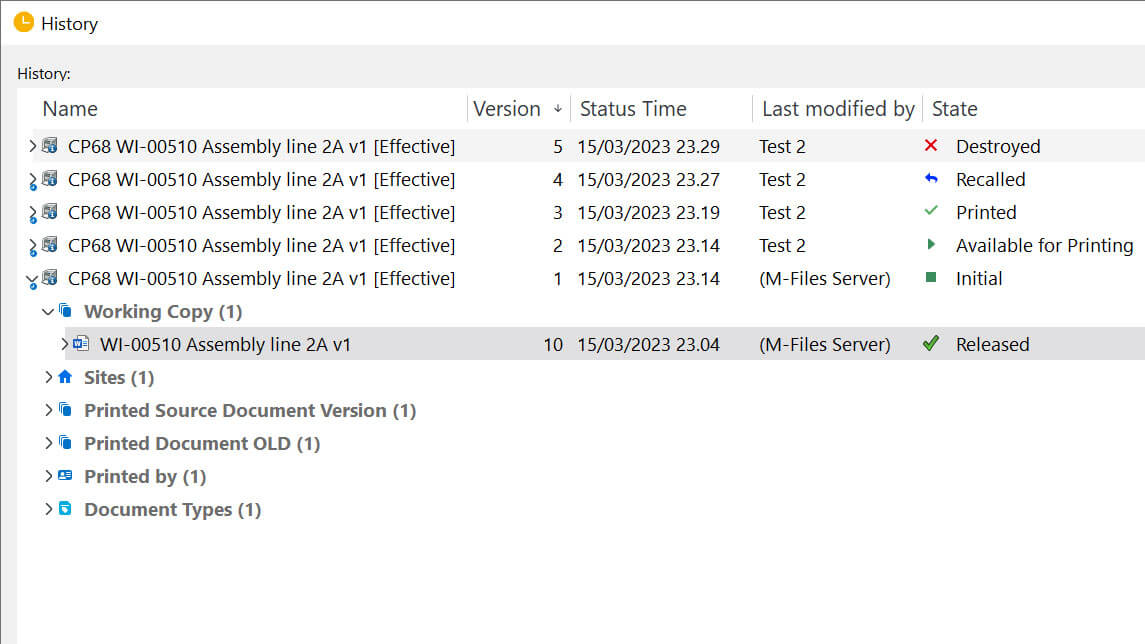
All controlled printouts will automatically be recalled when a new document version is released. Document-responsible persons, such as author, reviewer, and approver, can also issue recalls if needed or send out notifications about an upcoming recall.
After issuing a recall, all employees who printed the document would receive an automated email notification about an assignment to destroy the printed item and mark it as destroyed.
For Change Requests and CAPAs, all existing printouts need to be recalled and marked as destroyed before a Change Request or CAPA can be approved, and related documents are released in a new version.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Complete eQMS Software for Life Sciences
Document Management
Streamline document creation, approval, version control, and storage tasks using a robust document management solution.
Change Control
Control document modifications and ensure your company QMS complies with regulations and standards.
Audit Management
Manage and schedule various audits, from internal to external, supplier, and regulatory, within a single system.
Training Management
Monitor employee training and verify that all staff members are up-to-date with the most recent procedures.
CAPA Management
Facilitate the resolution of quality events by effortlessly creating and managing corrective and preventive actions.
Complaint Management
Mitigate quality risks and improve product quality by efficiently addressing customer complaints.
Frequently Asked Questions
What Is Controlled Printing?
Controlled printing allows users to create controlled prints of documents as well as track, manage and recall documents that have been printed or downloaded.
Controlled printout items contain a unique ID, date, creation time, responsible person, and print type in the footer on all document pages. This enables users to quickly identify different document copies.
In addition, the controlled printing feature can automatically recall prints when new document versions are released to ensure outdated prints are destroyed.
How Does the Controlled Printing Workflow Look Like In SimplerQMS?
In SimplerQMS, the workflow for controlled and uncontrolled printing involves the following steps.
First, the responsible person selects if the print is controlled or uncontrolled and enters a reason for printing, then a document printout item is generated.
The system records the date, time, print type, and person responsible for printing and generates a unique ID for the printout item. It also adds this information on the footer of the document.
Uncontrolled printouts have an additional “COPY” overlay in the header of the prints.
The document is then printed or downloaded and the user confirms if it was successful or failed, depending on the outcome.
When a document is updated and released in a new version, any related controlled prints will automatically be recalled and the responsible person will receive an assignment to mark them as destroyed with a deadline. Document-responsible persons can also send a recall notification of prints to all employees that printed the document.
This workflow helps to ensure that all printouts are managed and tracked, supporting compliance with regulatory requirements and maintaining data integrity.
How Can I Identify Which Copy of a Document I Have?
You can identify which copy of a document you have by checking the footer at the bottom of the document. It contains a unique ID, date, creation time, and name of the person responsible for printing, as well as the print type specifying whether it is a controlled or uncontrolled print.
Additionally, you can identify uncontrolled printouts by the “COPY” overlay in red at the top of the document which is not present in controlled printouts.
Is SimplerQMS FDA 21 CFR Part 11 and EU GMP Annex 11 Compliant?
Yes, SimplerQMS is compliant with FDA 21 CFR Part 11, which sets the guidelines for electronic signatures and electronic records. The system also complies with EU GMP Annex 11, which provides good manufacturing guidelines for computerized systems.
What is the Price of the SimplerQMS Solution with Controlled Printing?
Controlled printing is a feature included in the quality management software solution by SimplerQMS. By acquiring SimplerQMS, get access to the controlled printing feature as a part of your complete QMS software solution.
The prices only depend on the number and the types of licenses you purchase.
We provide a solution that includes all Life Science QMS modules, implementation, user training, and ongoing support.
For further information regarding our license types, features, and services included, visit our pricing page.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.