Form and Template Management for Life Sciences
Streamline your quality documentation processes and simplify document creation by efficiently managing forms and templates.
TRUSTED BY
























Form and Template Management In a Complete eQMS
SimplerQMS allows you to simplify the creation and management of all forms and templates and supports compliance with Life Science requirements, such as GxP, ISO 13485:2016, FDA 21 CFR Part 820, FDA 21 CFR Part 11, EU GMP Annex 11, EU MDR and IVDR, and others.
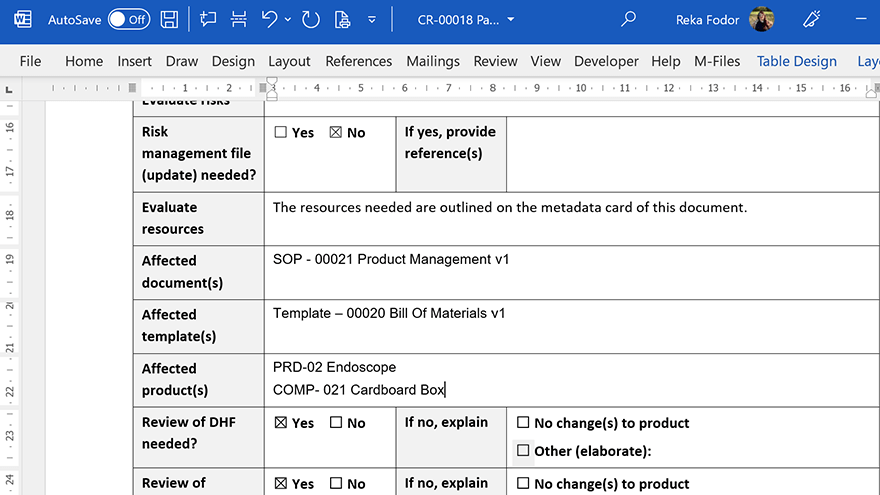
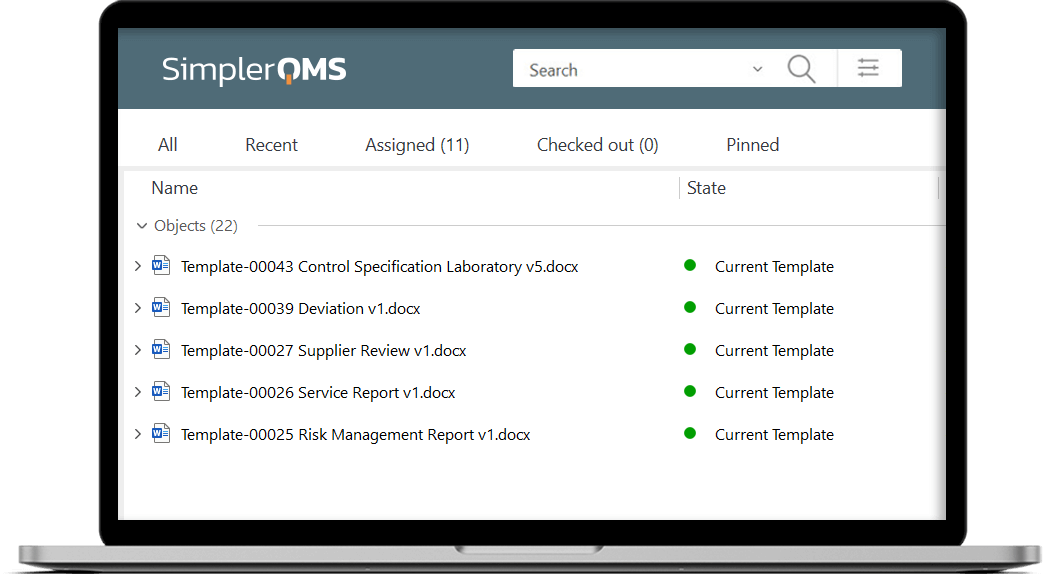
Streamline the process of creating documents using master templates and forms, and automatically include the required information in specified document fields. Use the pre-configured workflow to review, release and automatically retire old versions of forms and templates.
Form and template management is an integral capability of our complete eQMS software solution with all Life Sciences QMS modules, including document control, change management, training management, complaints management, CAPA management, audit management, and more.
Work Seamlessly With Microsoft Office Applications
Work on your documents using familiar Microsoft Office applications, such as Word, Excel, and PowerPoint.
Keep using your existing forms and templates, or use our complimentary package which includes the most commonly used forms and document templates in the Life Science industry.
Effortlessly build new forms and templates, for instance, for change requests, supplier qualifications, audit reports, CAPA forms, and more.
Enable Efficient Workflows and Automated Processes
Automate processes and streamline workflow efficiency by automatically naming, numbering, and versioning documents to save time and improve documentation structure.
Follow pre-configured workflows to create, review, and approve new forms and templates to ensure all information is properly addressed.
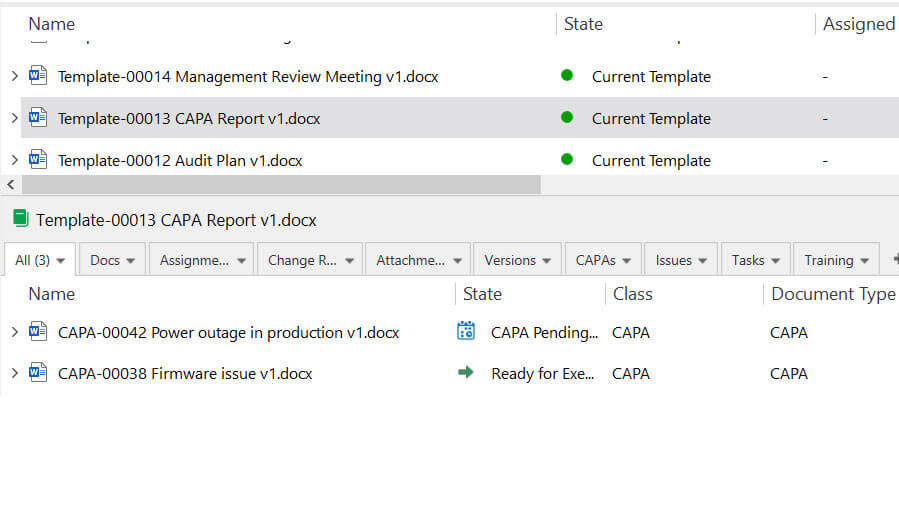
Use streamlined workflows that ensure that only the current version is available. This eliminates the stress of having multiple versions of nearly identical forms and templates and enables easy retrieval when needed.
Ensure Compliance with Life Sciences Requirements
Our complimentary form and template package is based on Life Sciences requirements. It can be used as a source of inspiration to create your own forms and templates, or you can use it as-is with minimal adjustments to fit your company’s needs.
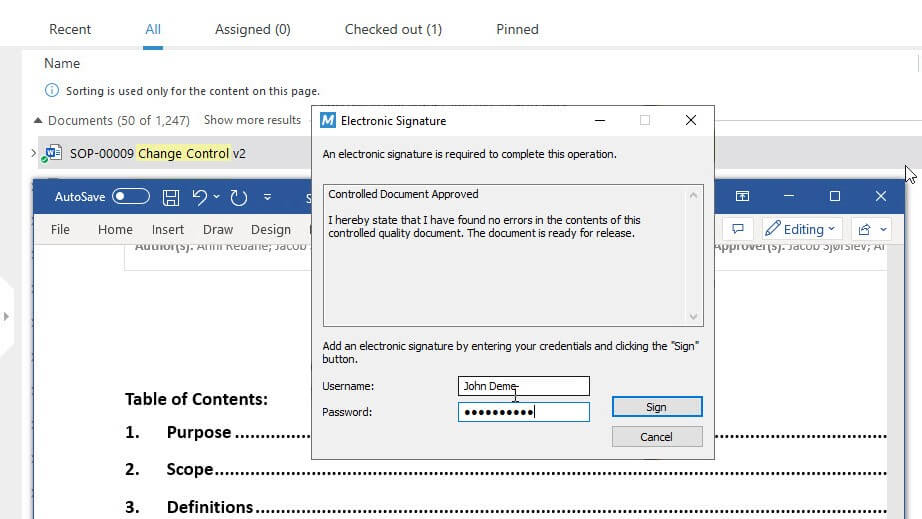
SimplerQMS complies with FDA 21 CFR Part 11, which sets the guidelines for electronic signatures and electronic records, and EU GMP Annex 11, which provides good manufacturing guidelines for computerized systems.
The system automatically records any document change and records data in a time-stamped audit trail for complete traceability.
Link Records to Forms and Templates
Link records to the most recent version of your forms and document templates and ensure they are always up to date.
This also helps you locate documents more quickly when needed and ensure the traceability of information.
For example, you can link your risk management procedure to a corresponding risk management report template or connect your CAPA procedure to the CAPA form.
Or you can easily see which records were created from each template or form.
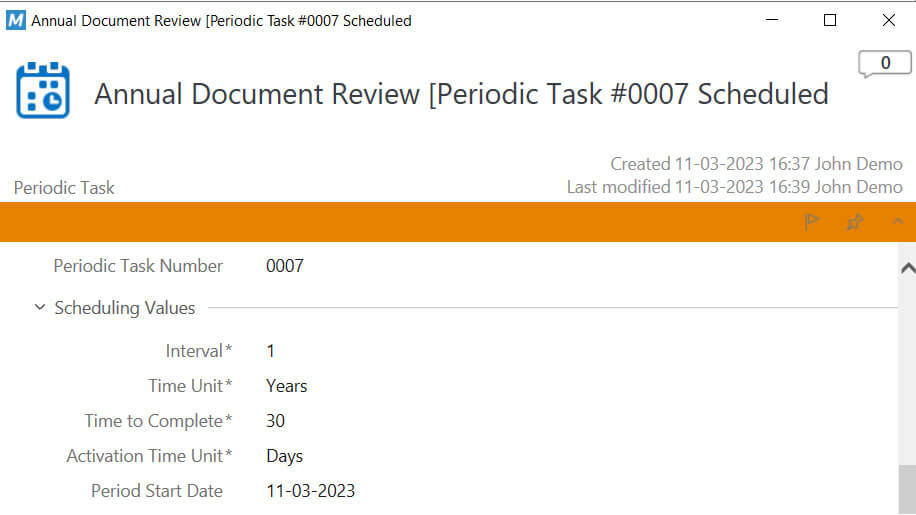
Set Periodic Document Review Reminders
Easily define dates for periodic document reviews and have automated reminders and notifications to ensure tasks are performed in a timely manner.
Email reminders will automatically be sent to the relevant employees before the scheduled review.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Complete eQMS Software for Life Sciences
Document Management
Consolidate document creation, approval, version control, storage, and other processes into one robust documentation solution.
Change Management
Manage document changes and ensure compliance of your company’s QMS with regulations and standards.
Training Management
Keep track of employee training and ensure all staff members are up-to-date with the latest procedures.
CAPA Management
Efficiently implement preventive and corrective actions to identify and mitigate risks.
Audit Management
Schedule and manage various internal, external, supplier, and regulatory audits using a single system.
Supplier Management
Efficiently manage suppliers and streamline supplier-related activities.
Frequently Asked Questions
What is Form and Template Management?
Form and template management is the process of creating, reviewing, approving, updating, and retiring document templates and forms, such as a Non-Conformance Report (NCR) template, CAPA form, and others.
It also involves making the latest version of the forms and templates available, linking it to relevant procedures, and facilitating its quick retrieval.
Such a process enables companies to maintain the consistency and accuracy of their forms and templates, secure them, streamline workflows, and help achieve compliance with Life Science requirements.
What Are Popular Form and Template Management Features?
Some of the most popular features are:
- Customizable forms and templates: have the ability to create and customize templates for different types of documents and forms based on the company´s specific needs.
- Version control: control versions of your documents over time and roll back to previous versions if necessary.
- Electronic signatures: use electronic signatures to document responsibilities and justifications for actions performed.
- Automatic notifications: set up automatic notifications to alert personnel when a task is assigned to them.
- Search and retrieval: have the ability to search and retrieve documents using keywords in document titles and content.
- Integration with other applications: use already familiar Microsoft Office applications, such as Word, Excel, PowerPoint, Outlook, etc.
- Pre-configured workflows: follow workflows based on Life Science requirements for quality documents, change requests, and more.
How Does the Form and Template Management Workflow Look Like?
The first step is the form or template creation using SimplerQMS’s complimentary package or uploading an existing form or document template from your local drive using drag-and-drop functionality.
To provide more data security, controlled document templates or forms require change requests to be modified by default in the system. But authors and responsible persons can define whether the document needs change requests to be updated or not.
After drafting the form or template, you can send it to the appropriate personnel within the workflow for review as many times as needed. It is also possible to modify the reviewers’ list or even exclude the review process altogether.
Then, you can send the final document draft for approval and release. The template or form can become effective on a specific date or immediately.
SimplerQMS automatically sends notifications and reminders to notify the relevant people when it requires review or approval actions.
Finally, you can retire forms and templates that are no longer in use.
Our Knowledge Base article about managing templates provides further information on the workflow for you to learn more.
Is SimplerQMS FDA 21 CFR Part 11 and EU GMP Annex 11 Compliant?
SimplerQMS complies with both FDA 21 CFR Part 11, which outlines the standards for electronic signatures and electronic records. As well as, EU GMP Annex 11, which sets out good manufacturing practices for computerized systems.
What is the Price of the SimplerQMS with Form and Template Management?
The pricing of SimplerQMS software is determined by the quantity and types of licenses you acquire.
SimplerQMS is a complete eQMS software solution that includes all Life Science QMS modules and features for one subscription price. Things like implementation, user training, hosting, and ongoing support, are all included, so you can be sure that once you start using the solution, there will be no surprise costs.
For further information regarding our license types, features, and services included, visit our pricing page.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.