QMS Software for Medical Devices & MedTech Industries
Facilitate quality management processes while maintaining regulatory compliance with purpose-built medical device QMS software.
TRUSTED BY
























Streamline Your Medical Device QMS and Ensure Regulatory Compliance
Medical device manufacturers are required to meet rigorous quality standards, regulations, and guidelines such as ISO 13485, the Quality System Regulation or QSR (FDA 21 CFR Part 820), the Current Good Manufacturing Practices (GMP), and others.
Quality management software by SimplerQMS streamlines medical device quality management systems and supports compliance with these and many more global medical device regulations and standards.
Connect All Your Quality Processes
Easily integrate your quality processes such as audits, training management, design control, non-conformances, complaints, CAPAs, change control, suppliers, and others.
Upload and link any document, record, file, or email to any quality process for better traceability. Access a centralized, cloud-based repository for all your quality data and documents that can be accessed anytime, anywhere, and presented to the auditor in a few clicks.
Work With Familiar Microsoft Office Tools
SimplerQMS quality management software integrates with familiar Microsoft Office applications such as Word, Excel, PowerPoint, and Outlook so that you can work with the tools you’re already familiar with.
Edit the document and save it in SimplerQMS Cloud with just a few clicks. No need to download and upload files manually or learn new document editing software.
Automate Document Control Processes
Create and manage documents and records from a central location.
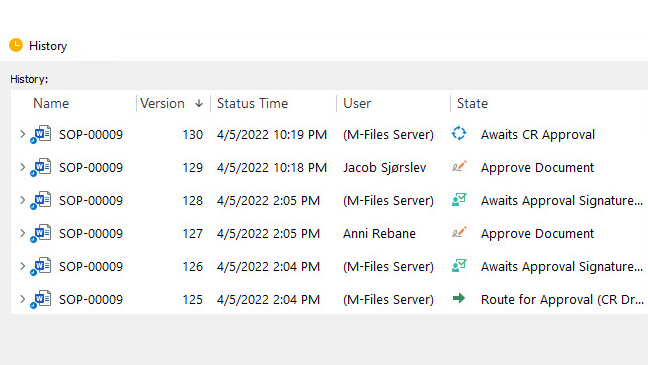
Automatically route documents for approval, ensure that all stakeholders have access to the most current versions, and track document history for complete traceability.
With features such as 21 CFR Part 11 compliant electronic signatures, automated data collection, version control, time-stamped audit trails, escalation of overdue activities, and email notifications, you can be sure that your document control processes are under control.
Train Employees Effectively
With the medical device eQMS software solution by SimplerQMS, you can create and deliver training to your employees quickly and easily, and ensure that all employees are trained on the most current procedures and policies.
Upload training material in video, PowerPoint presentation, PDF, or other formats.
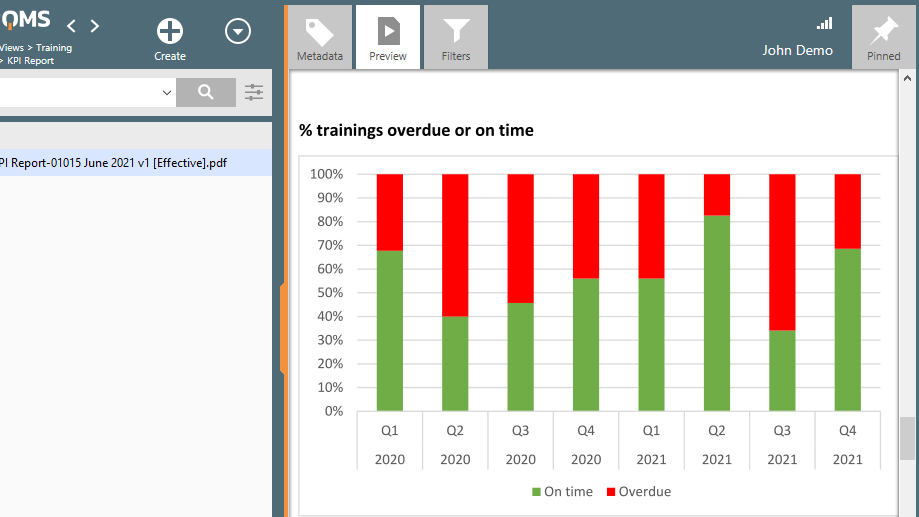
Assign training to employees and track their progress. Create training assessments to test employee knowledge. Generate training reports to show compliance status.
Maintain Audit-Ready Design Control Documentation
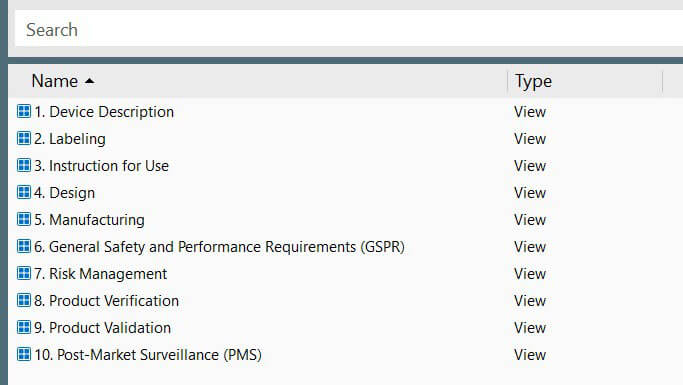
Track and manage your design control processes from design planning to design transfer and commercialization in one location.
Relate the same design control document and records to multiple archives, for instance, Technical File (TF), CE Marking, 510K Submission, Design History File (DHF), etc.
Get an overview and quick access to the necessary documentation through configurable and customizable dashboards.
Manage Various Quality Events in One Place
Detect, verify, investigate, and correct various quality events such as audit findings, non-conformances, customer complaints, and CAPAs in one place.
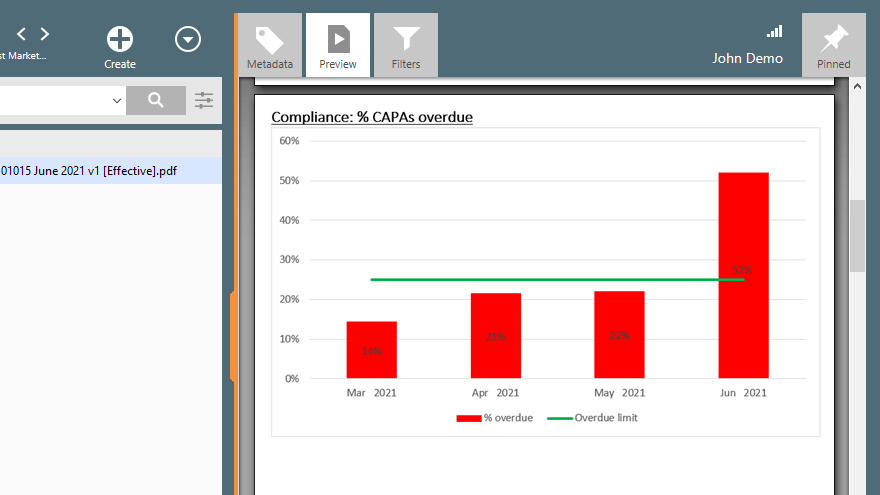
Utilize pre-configured workflows, best-practice forms, and automated escalation of overdue activities to ensure that nothing falls through the cracks and that all quality events are properly resolved in a timely manner. Track and trend your quality metrics to identify areas for improvement.
Avoid Time-Consuming Validation Processes
SimplerQMS quality management system (QMS) solution is fully validated according to GAMP5. The system fully complies with ISO 13485:2016, 21 CFR Part 11 and 820, and the EU Annex 11 regarding validation and electronic signatures.
Plus, we perform continuous re-validation of the software on a monthly basis.
This means that your organization does not have to spend any time or money on system validation processes.
What Validation Documentation Evidence Does SimplerQMS Provide?
The validation documentation evidence includes:
- Validation Procedure
- Validation Plan
- Validation Report
- IQ, OQ and PQ Documentation
100% Transparent Pricing
With SimplerQMS you get everything for one subscription price, depending on the number of licenses.
This means there are NO implementation fees, extra charges for hosting, system validation, user training, ongoing support, or additional modules. It’s that simple.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
All-in-One QMS Software for Medical Device Organizations
Document Control
Generate, approve, and control quality and regulatory documentation from a central location.
Design Control
Track and manage your design control processes from design planning to commercialization.
Employee Training
Create and manage employee training programs and track the progress of each trainee.
Non-Conformances
Record, evaluate, analyze and manage non-conformances throughout your organization.
CAPA Management
Prevent quality issues from happening again with effective CAPA processes.
Risk Management
Consolidate risk and manage your risk management file in a well-organized manner.
Complaint Management
Efficiently track, manage and resolve customer complaints in a timely manner.
Audit Management
Schedule and track internal and external audits, and manage audit findings.
Supplier Management
Streamline supplier quality management processes from qualification to performance monitoring.
Frequently Asked Questions
What Is Medical Device Quality Management Software?
Medical device QMS software is a computer application that helps medical device organizations manage their Quality Management System (QMS) in a more efficient and compliant manner.
It allows users to store quality-related documents and records in a central location, making it easier to access and share information between different departments and team members. Comprehensive medical device eQMS solution like SimplerQMS covers all aspects of quality management, from document control, to change control to design control, non-conformance management, CAPA management, supplier management, and much more.
What Are the Benefits of Using an eQMS for Medical Device Companies?
The main benefits of using eQMS software for medical device manufacturers are increased efficiency in managing quality-related processes, lower risk of quality issues, reduced costs (especially administrative), easier compliance with regulatory requirements, better visibility into quality metrics, improved collaboration between different departments, and improved customer satisfaction.
Is SimplerQMS Medical Device QMS Software Validated?
Yes, SimplerQMS is a fully validated QMS solution according to GAMP5 and complies with ISO 13485:2016, 21 CFR Part 11 and 820, and the EU Annex 11 regarding validation and electronic signatures. We also conduct continuous re-validation taking away the need for our customers to spend any time or money on system validation.
How Much Does SimplerQMS QMS Platform Cost?
SimplerQMS offers an all-in-one eQMS platform that includes all software modules, hosting, validation, implementation, user training, and ongoing support for one subscription price.
That being said, there are no implementation fees, extra charges for hosting, system validation, user training, ongoing support, or additional modules.
The price is based on the number of licenses you need. To learn more about SimplerQMS and get a custom quote, please book a demo.
How Much Time Does It Take to Implement SimplerQMS QMS Solution?
Based on our experience, it takes most customers about 6 weeks to implement SimplerQMS.
The exact time frame will depend on the size and complexity of your organization, as well as the number of documents that need to be migrated into the system.
Our team of experts will work closely with you throughout the implementation process to ensure a smooth and successful transition to our medical device quality management software.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To learn how you can make the most of SimplerQMS in your medical device company – book a tailored demo.