21 CFR Part 11 Compliant Document Management System
Effortlessly handle electronic records and electronic signatures through streamlined document workflows and ensure compliance with the FDA’s 21 CFR Part 11 regulations.
TRUSTED BY
























21 CFR Part 11 Compliant DMS Within a Complete eQMS
With SimplerQMS you can easily create and manage electronic records while utilizing electronic signatures to sign documents with the same level of trust and legitimacy as handwritten signatures.
Use our pre-configured workflows based on Life Science requirements to ensure that documents and records are properly managed. The system will automatically prompt you to use electronic signatures to sign off, documenting responsibilities and justifying the actions taken.
SimplerQMS is not just a Document Management System (DMS), we provide a complete eQMS solution compliant with 21 CFR Part 11 and other Life Science requirements. The system includes all Life Science QMS modules such as document control, change management, training management, complaints management, CAPA management, audit management, and many others.
Control User Access with Secure User Access Controls
Prevent unauthorized access to information by defining which roles or specific personnel are granted access to relevant documents.
Safeguard your data with more robust security measures, such as two-factor authentication and periodic password resets, to ensure data privacy and confidentiality.
SimplerQMS uses Microsoft Entra ID (previously known as Microsoft Azure Active Directory), a cloud-based identity, and access management service, to manage license assignments and system access.
Streamline Your Work with Microsoft Office Integration
Keep working with familiar Microsoft Office applications such as Microsoft Word, Excel, and PowerPoint. Create, edit, and collaborate on a document and save it with one click in SimplerQMS Cloud.
Improve productivity using our pre-configured workflows to streamline document authoring, review, approval, and editing processes.
SimplerQMS also offers a complimentary form and document template package based on Life Science requirements as well as form and template management capabilities to simplify document creation.
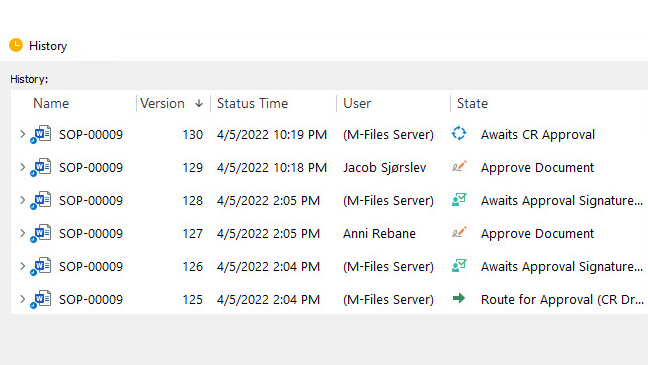
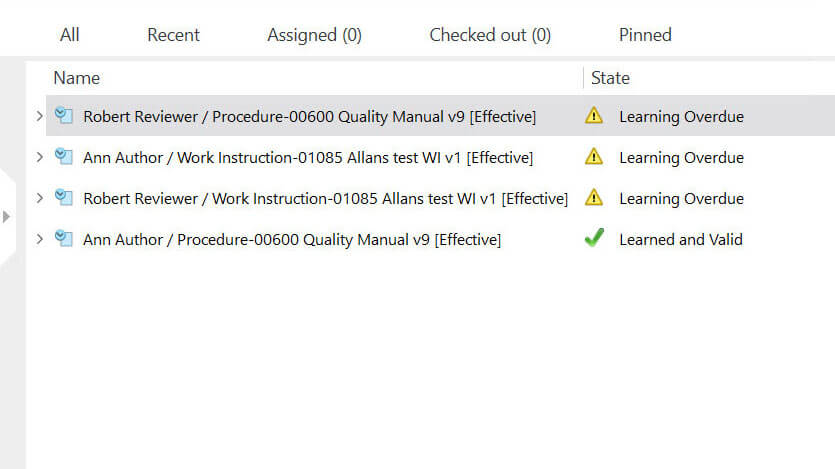
Keep Accurate Records with Time-Stamped Audit Trail
Use secure, computer-generated, time-stamped audit trails to automatically record the date and time of actions related to any change to the document.
SimplerQMS software allows you to easily access audit trails to overview complete document history at any time, for example during an audit situation.
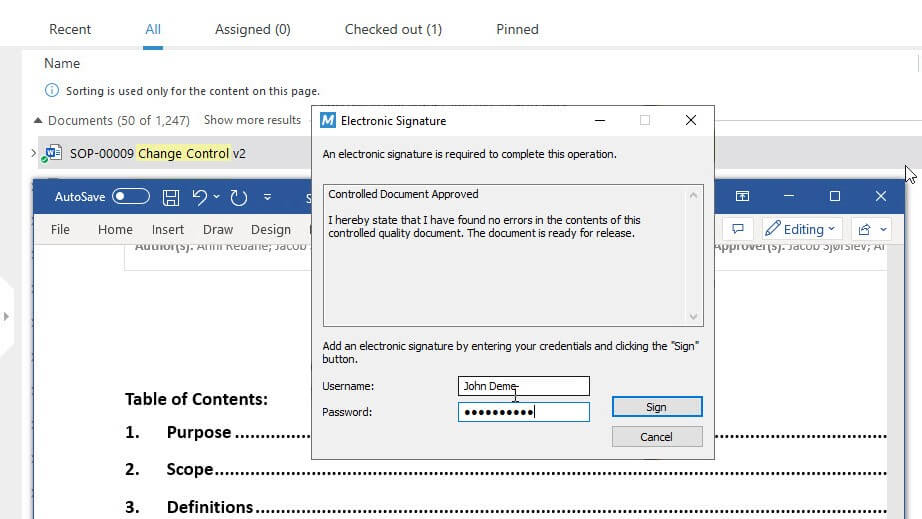
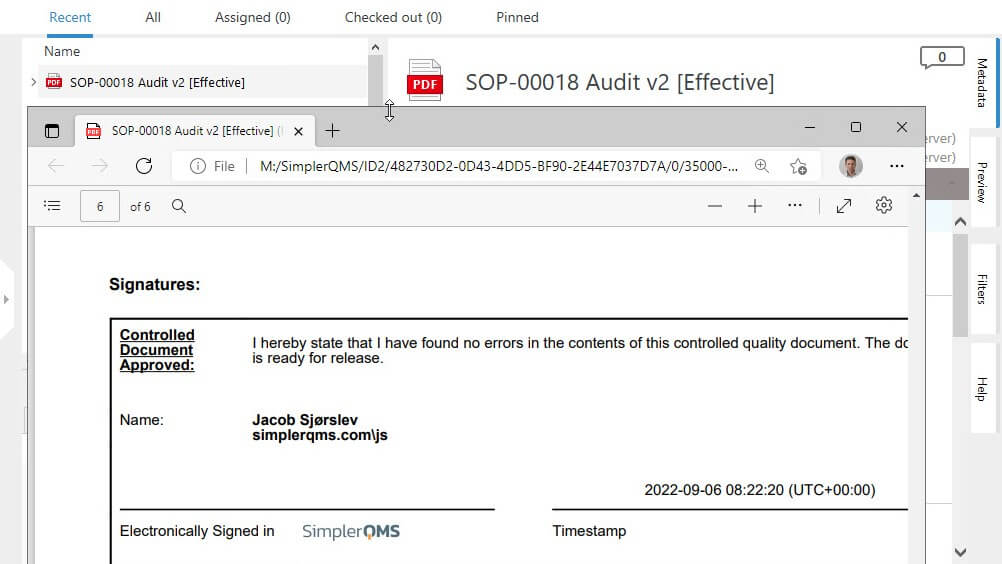
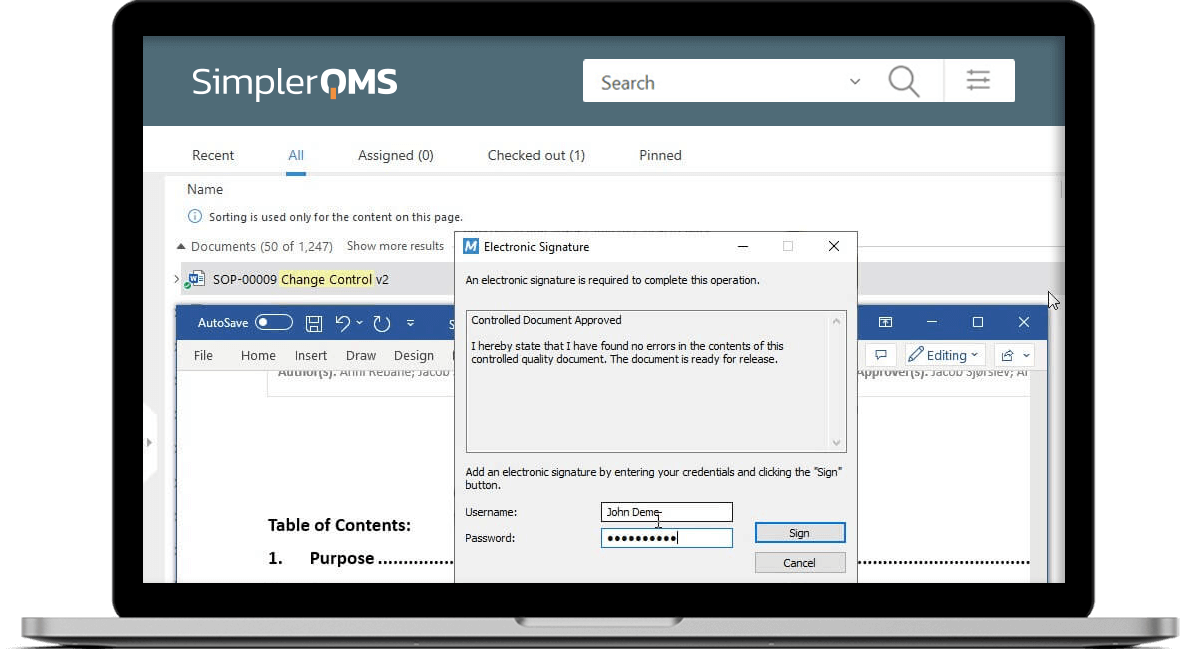
Sign Documents Using Electronic Signatures
Ensure unique electronic signatures for each employee using unique passwords.
Sign off documents and have all electronic signature data automatically recorded, including employee name, date and time, and the meaning of the signature.
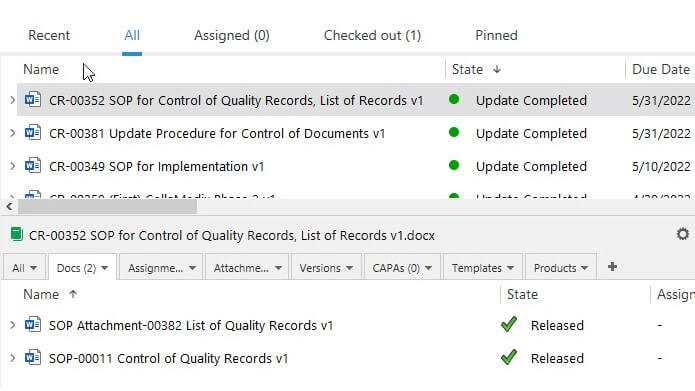
Maintain Control with Document Version Control
Have a complete list of different versions of the document. Compare older document versions and roll them back if necessary.
Prevent unauthorized changes to documents by implementing change requests on specific document types, and much more.
Save Time with Automated Workflows
Automate document routing, notifications, reminders of upcoming activities, and periodic tasks, such as periodic document reviews.
Use automated workflows to streamline the documentation process. Reduce time to create, review, approve, and manage documents.
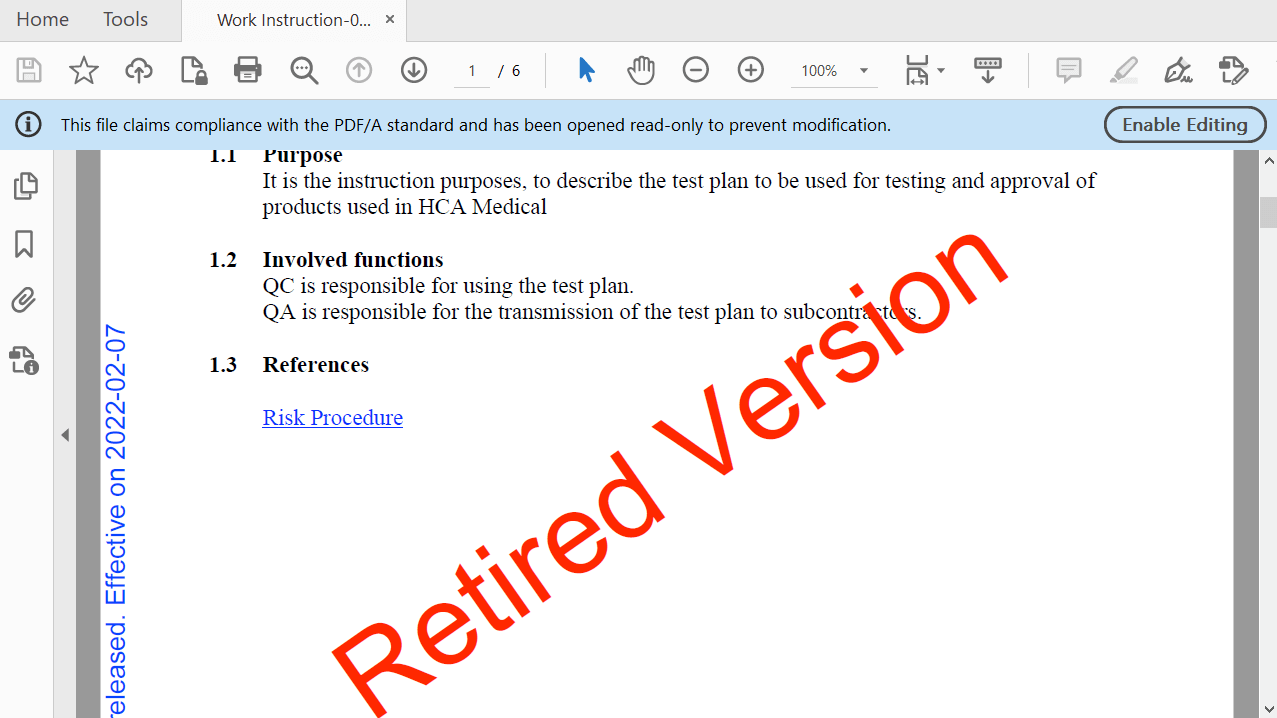
Streamline Document Archiving and Retrieval Processes
Search for keywords in the document title and content for quick and easy document retrieval at any time during the document retention period.
Facilitate the archiving of documents – automatically. When a new version of a document is released, the previous one is automatically stamped with a watermark for easy identification, retired, and archived.
Integrate to Other Systems
Centralize data from different sources to prevent data silos and avoid the loss of important information.
SimplerQMS easily interlinks with all QMS modules allowing for seamless workflows. For example, escalate customer complaints to the CAPA process while relating all the relevant documents to one another.
You can also integrate existing systems in your company such as CRM, ERP, LMS, MES, EBRS, CMS, and PLM with our eQMS, using open API (Application Programming Interface).
Ensure Compliance with Fully Validated Electronic System
The system is fully validated according to ISPE GAMP5 and is re-validated upon the creation of a new version or upon applying standard updates. This allows you to avoid spending any time on system validation.
Have peace of mind with a system that complies with FDA regulations, including 21 CFR Part 11, FDA 21 CFR Part 820, and other relevant regulations and standards such as EU GMP Annex 11 and ISO 13485:2016 when it comes to the validation of computer software used for your quality management system.
Streamline Training Management with Automated Retraining Assignments
Streamline the training process by automating training-related task assignments, notifications, and reminders to ensure training is completed on time.
Achieve compliance with training requirements by ensuring that all employees who use an electronic system for managing documents have the knowledge to perform their assigned tasks.
Link Electronic Signatures to Corresponding Electronic Records
Link electronic signatures to their respective electronic records to ensure complete traceability and accountability of actions.
Electronically sign documents and automatically record the signer’s name, date, time, and the meaning of the signature on signed electronic records.
See Electronic Signatures and Workflows in Action
Watch this brief video to see how SimplerQMS simplifies document signing with 21 CFR Part 11 compliant eSignatures.
You will also see the capabilities such as automated workflows, notifications, and reminders of upcoming activities, and how they make document management simpler and more efficient for both internal and external users.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Beyond Just Document Management Solution
Change Management
Ensure compliance and maintain structure in your QMS by managing all changes appropriately.
Training Management
Create training plans, automate notifications, and track progress to ensure efficient employee training.
Audit Management
Make audit management processes more efficient and less time-consuming.
Supplier Management
Simplify tasks related to suppliers and manage their documentation as per the requirements.
Nonconformances
Record, manage and resolve nonconformances effectively.
CAPA Management
Identify, address, resolve, and report all the necessary preventive and corrective actions.
Frequently Asked Questions
What is a 21 CFR Part 11 Compliant Document Management System (DMS)?
A 21 CFR Part 11 compliant DMS is a software solution that complies with the regulations outlined in Part 11 of Title 21 of the Code of Federal Regulations (21 CFR Part 11). This type of software helps companies securely manage their documents, control access to confidential data, and authenticate user actions such as document signing.
These regulations were established by the US Food and Drug Administration (FDA) to define the criteria under which electronic records and signatures are considered trustworthy, reliable, and equivalent to paper records.
For pharmaceutical, biotech, medical device, and other Life Science companies, having a document management system that is compliant with 21 CFR Part 11 is critical for maintaining compliance with FDA regulations and ensuring the integrity and security of electronic records and signatures.
Who Needs to Use a 21 CFR Part 11 Compliant Documentation System?
21 CFR Part 11 complaint DMS is necessary for pharmaceutical, biotech, medical device, and other Life Science companies that must comply with Title 21 of the Code of Federal Regulations (21 CFR Part 11).
In other words, any company that is subject to FDA regulations and requirements that manages records in electronic form (creates, modifies, maintains, archives, retrieves, or transmits, under any records requirements set forth by the FDA) must use a 21 CFR Part 11 compliant document management solution for that purpose.
What are the Most Popular Features of a 21 CFR Part 11 Compliant DMS?
Some of the most popular features of a Document Management System include user access controls, electronic signatures, time-stamped audit trails, document versioning, automated workflows, cloud-based storage, data integrity, and backup security measures.
Does a Document Management System Need to be Validated?
Yes, according to 21 CFR Part 11, document management systems must be validated to ensure accuracy, reliability, consistent intended performance, and the ability to identify invalid or altered records.
SimplerQMS solution is fully validated according to ISPE GAMP5.
Our software complies with other regulatory requirements regarding validation, such as EU GMP Annex 11, 21 CFR Part 11, 21 CFR Part 820, and ISO 13485:2016.
How Much Does Part 11 Compliant Document Management Software Cost?
The cost depends on the type and number of licenses you acquire.
SimplerQMS includes all QMS modules, hosting, validation, implementation, user training, and ongoing support for one subscription price, so there are no additional fees.
We provide not just a robust document management solution but a complete eQMS platform with interlinked QMS modules for document management, training management, change management, audit management, customer complaints, supplier management, and much more.
Please visit our pricing page to learn more about SimplerQMS and get a custom quote.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.