Complaint Management Software for Life Sciences
Streamline your complaint management workflow and turn complaints into product improvement opportunities and increase customer satisfaction.
TRUSTED BY
























Effective Complaint Management Within a Complete eQMS
Customer feedback and complaint management are important components of post-market surveillance.
The SimplerQMS solution helps Life Science companies operating in industries such as pharmaceuticals, biotechnology, medical device, and others, to document and manage all complaints processes in one secure cloud-based system.
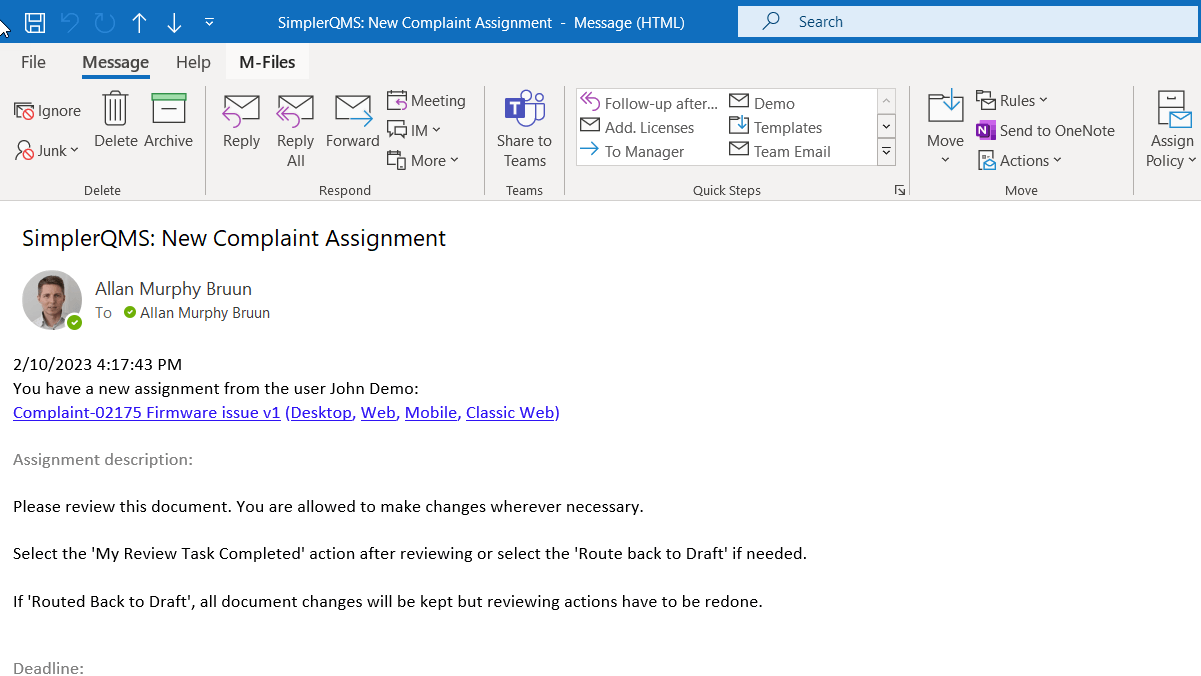
The system offers features such creation of complaints using templates, relating complaints to customers, products, LOTs, and external documents, assigning Issue Handlers, setting reminders for tasks, escalating issues to CAPA (if necessary), and more.
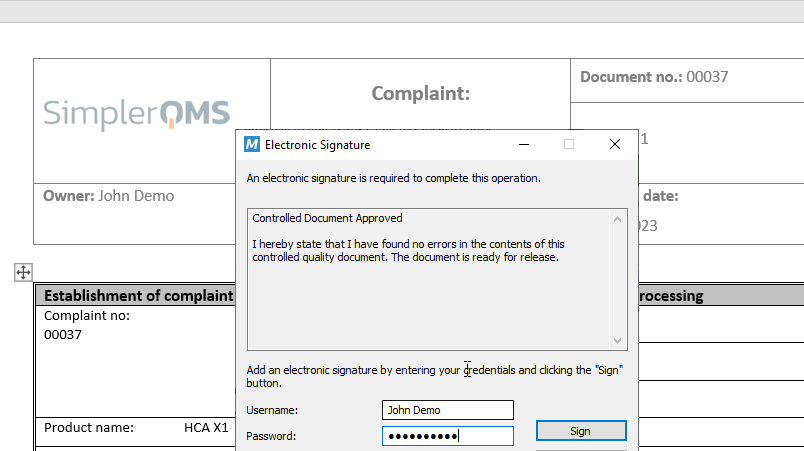
SimplerQMS Complaint Management software is part of an integrated quality management solution, which includes all core QMS modules such as document control with electronic signatures, change management, training, equipment management, CAPA, audit management, and much more.
Easily Capture Customer Feedback and Complaints
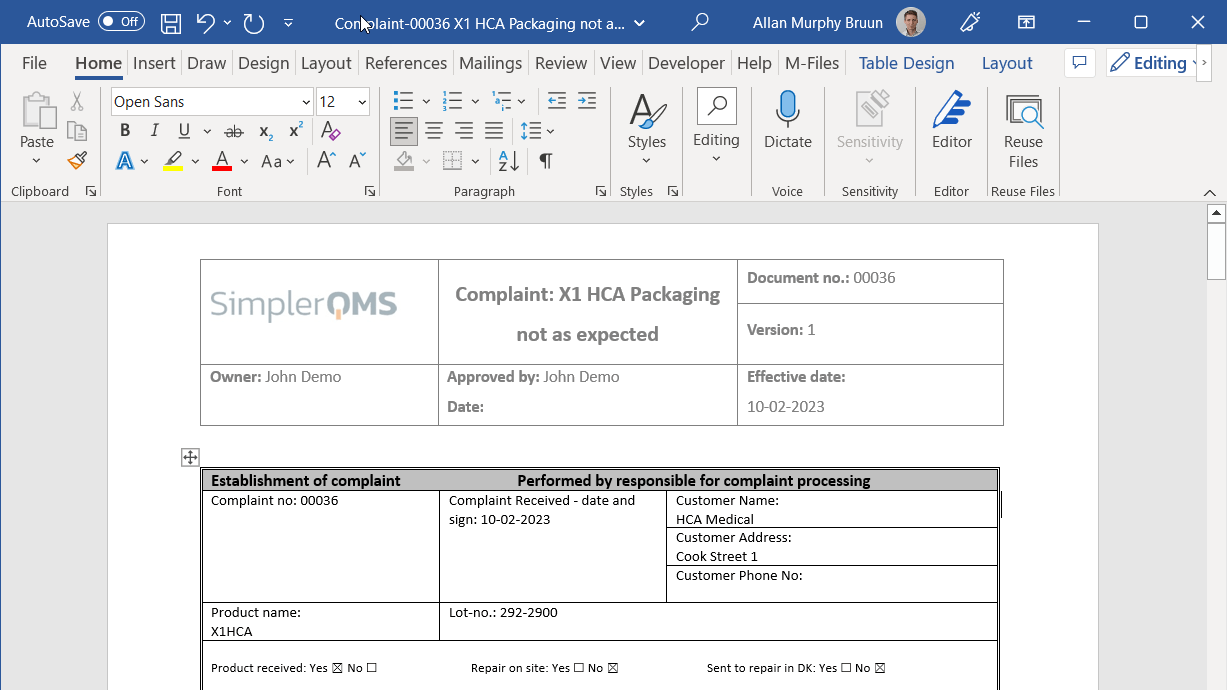
Create complaint documents related to customers, suppliers, or products. Fill out the metadata card and have all relevant information automatically linked to the complaint.
Easily import your own complaint templates or use one of the complementary templates from our template package. Templates and forms can be customized or created according to your needs.
Keep using the already familiar Microsoft Office Word, Excel, and PowerPoint applications to work on your templates and complaints.
Manage All Complaint Activities in One Place
Store all documents in one place using a cloud-based system and easily access the files from anywhere.
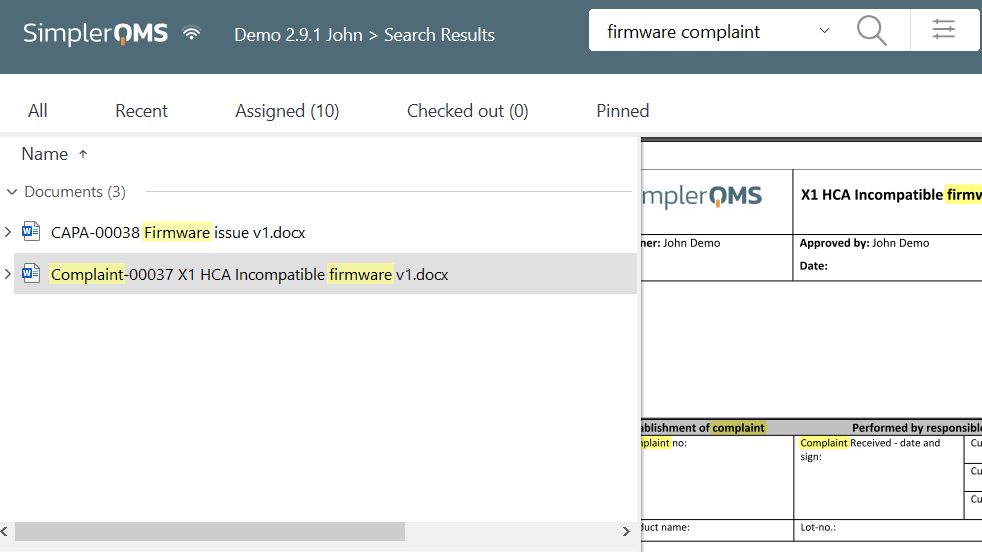
Search specific complaint-related documents in the system by matching keywords in the title and/or content of the documents. This feature also makes it easier to retrieve necessary documents during an audit.
Link complaint documents to products, suppliers, customers, regulations chapters, and more with a few simple clicks.
Monitor Complaint Status Effectively
Have an overview of complaint status using highly customizable views.
Automatically record all document modifications and justifications in a complete, time-stamped audit trail.
Analyze trends using complaint-specific KPI reports. For example, analyze the total number of open and closed complaints in specific periods, the number of complaints by severity, product, department, and more.
Export data from a view to a local device for further, more in-depth analysis, if necessary.
Escalate Complaints to CAPAs as Needed
Assign the responsible people to specific tasks and fill in all workflow information in the metadata card.
Easily define the Issue Handler – this workflow participant is responsible for deciding if a CAPA is necessary to resolve a complaint.
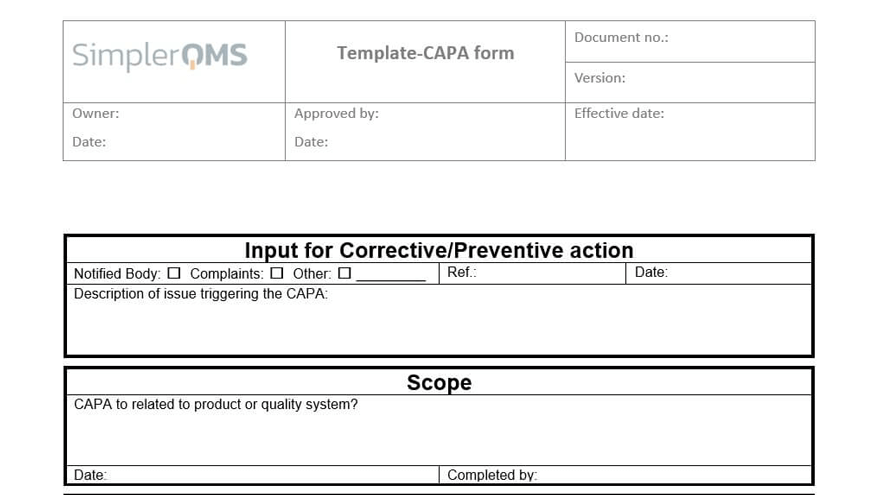
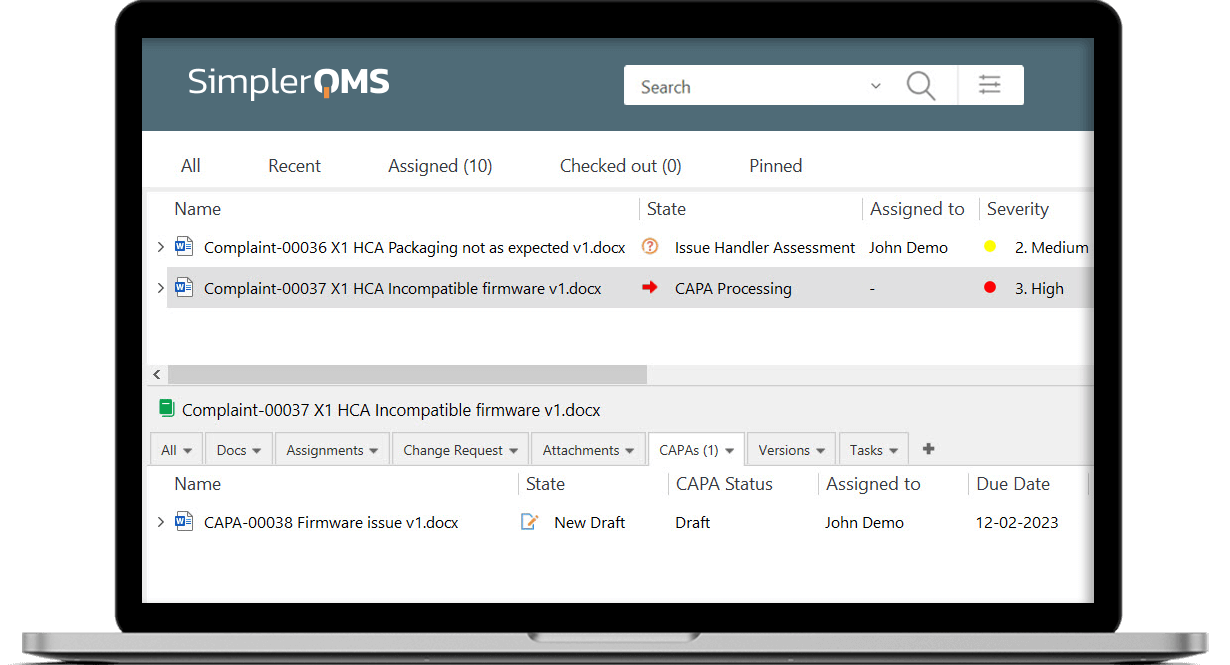
Escalate a complaint directly to a CAPA, if necessary, and have them automatically linked.
The system supports the CAPA management process, from creating a new CAPA, attaching evidence, and relating relevant documents, to scheduling effectiveness checks.
Automate Complaint Management Activities
Create complaint-related assignments and automate notifications for required actions before due dates.
Set up activities and their due dates, and the system will automatically send reminders to the assigned personnel and help ensure that tasks are completed on time.
When escalating a complaint to a CAPA, the system will automatically create a periodic task to assess the effectiveness of the CAPA.
Connect All Your Processes
Manage all quality processes in a single system. All QMS modules are included and interlinked in SimplerQMS.
Have at your disposal document control, change management, training, supplier management, CAPA, audit management, and more.
Integrate SimplerQMS with other applications, such as CRM through an open API, if necessary.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Beyond Just Complaint Management
CAPA Management
Keep issues from recurring by implementing preventative and corrective actions seamlessly.
Document Control
Create, approve, store, and version all documents using a state-of-the-art document control solution.
Equipment Management
Easily control and manage equipment, equipment maintenance, and calibration tasks.
Audit Management
Streamline audit management processes to improve audit assurance and productivity.
Supplier Management
Improve supplier overview and control by streamlining supplier-related activities.
Risk Management
Identify, analyze, and reduce hazards within your organization through effective risk management.
Frequently Asked Questions
What Is Complaint Management Software?
Complaint Management Software is a tool that allows you to capture, analyze, and handle customer complaints.
The software enables companies to automate activities related to the complaint-handling process, for instance, assignment notifications and reminders, helping ensure complaints are solved on time.
With such a digital solution, you can store all complaint-related documents in a single place and have them readily available during audits. Use customizable views for complaint monitoring and much more.
SimplerQMS Customer Complaints Management Software offers all these features and more. We provide a complete eQMS solution with all core QMS modules, such as document control, training management, change control management, audit management, nonconformance management, CAPA management, equipment management, and others.
How Do You Keep Track of Customer Complaints in SimplerQMS?
You can keep track of customer complaints by first creating a new complaint document in the system, filling in all related information, and setting relations in the metadata card.
If necessary, the system offers an easy route to escalate complaints to CAPAs and interlinking all documents to ensure traceability.
SimplerQMS automatically records all activities performed inside the system and provides a complete audit trail. You can monitor document status, have an overview of which actions were executed, by whom, exactly when, and much more.
How Can I Escalate a Complaint to a CAPA in SimplerQMS?
SimplerQMS solution allows you to escalate a complaint to a CAPA by clicking on the “Create CAPA” action inside the specific complaint view.
For instance, you can escalate a customer complaint to a CAPA as needed depending on the issue’s risk severity, reoccurring status, or financial impact.
Inside the SimplerQMS Software, the Issue Handler is responsible for deciding if a CAPA is necessary. In these cases, escalating a complaint to a CAPA is easy by clicking on the “Create CAPA” action.
The system automatically relates all documents and records changes in a time-stamped audit trail.
To learn more about how to manage complaints in SimplerQMS read our Knowledge Base article on managing recorder issues and CAPAs.
How Much Time Does Complaint Handling Software Take to Implement?
The SimplerQMS complete solution usually takes five to six weeks to be implemented.
The complaint management module is part of a complete eQMS solution. Implementation of document control, change management, and training management modules is required before complaint management module implementation can begin.
In addition, the time needed to implement the system can vary depending on the number of documents that need to be created or migrated and your company’s time resources.
How Much Does Implementing a Complaint Management Solution Cost?
The cost of the SimplerQMS Software is based on the number of licenses you acquire.
The license includes all QMS modules, training, ongoing support, validation, and hosting.
SimplerQMS includes all features and services in its subscription price, so there are no additional costs.
To learn more about the types of licenses and all the features and services included, please visit our pricing page.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.