Purpose
Learn how to:
- Create and update the following Recorded Issues: Complaints, Nonconformances/Deviations, Supplier Issues and Continuous Improvements
- Create CAPA and CAPA Attachments with the Recorded Issues
Expected Outcome
Users are equipped to handle all Recorded Issues (excluding results from Audit Findings), and CAPAs in SimplerQMS
Prerequisites for all
- Must be logged in to SimplerQMS
- Access to the site that the Recorded Issue is assigned to
- Must be a member of one of these ‘User Groups’ to view records:
- All internal and external users
- Must be a member of one of these ‘User Groups’ to author, review and approve records:
- All Contributors
Prerequisites for the Quality Assurance Responsible
- Must be a member of one of these ‘User Groups’
- Quality Assurance Responsible
Prerequisites for creating Recorded Issues
- Must be a member of one of these ‘User Groups’ to create new records:
- Masterdata Administrator
- Complaint Handler, Audit Handler and/or NC Handler
Prerequisites for creating CAPAs
- Must be a member of the ‘User Group’ to create new CAPAs:
- CAPA Handler (named Issue Handler on the Recorded Issue metadata card)
Section 1: Configuration: Recorded Issue List & Vigilance Reporting
Recorded Issue List
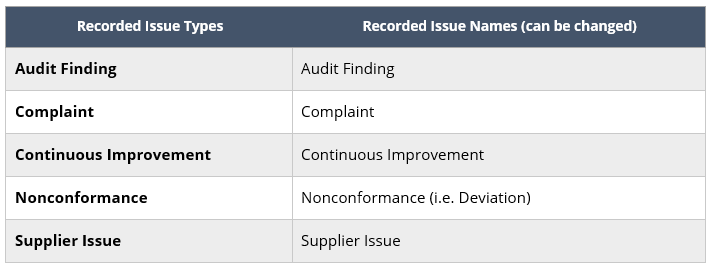
The recorded issue list can be configured based on the industry or preference of the general users of SimplerQMS. It is important to decide on how recorded issues are named as it will be applied on every record created after the configuration.
The user with a Metadata Administrator user group can make the configuration.
The default names are presented on the table:
Vigilance Reporting
As part of the configuration process, vigilance reporting can be set up. The system can be customized according to the company’s own process and preference.
Vigilance reporting is used for any issues that would need to be escalated to authorities the company complies with. If this feature is turned on, a two-step vigilance reporting feature will be enabled consisting of a Pre-assessment before deliberation for CAPAs (in case the report needs to be made urgently, this step ensures that reporting details can be captured early on) and a final assessment after the CAPA deliberation is completed (to finalize any missing details or provide details if not added beforehand). By default, Nonconformances and Complaints are set to Yes on vigilance reporting, while all other recorded issues are set to No.
The user with a Metadata Administrator user group can adjust the configuration.
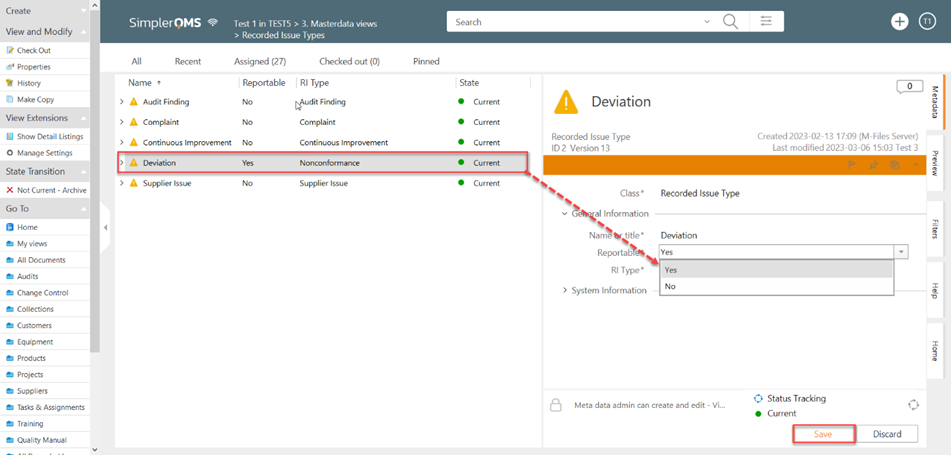
I. From Masterdata views, click on Recorded Issue Types
II. Select the recorded issue, In the metadata, Select Yes on the Reportable field
III. Click on Save.
NOTE: This configuration does not mean that you will have to report every case. Choosing Yes on reportability will ensure that you are asked every time that Recorded Issue Type is raised if reporting is required or not, to ensure that you follow procedure. In both steps of the reporting, you will be able to choose No on reportability per the individual document. Refer to section 4 & 8 of this WI.
For more details on setting up Recorded Issue Types: Refer to WI: Masterdata
Section 2: Workflow Review
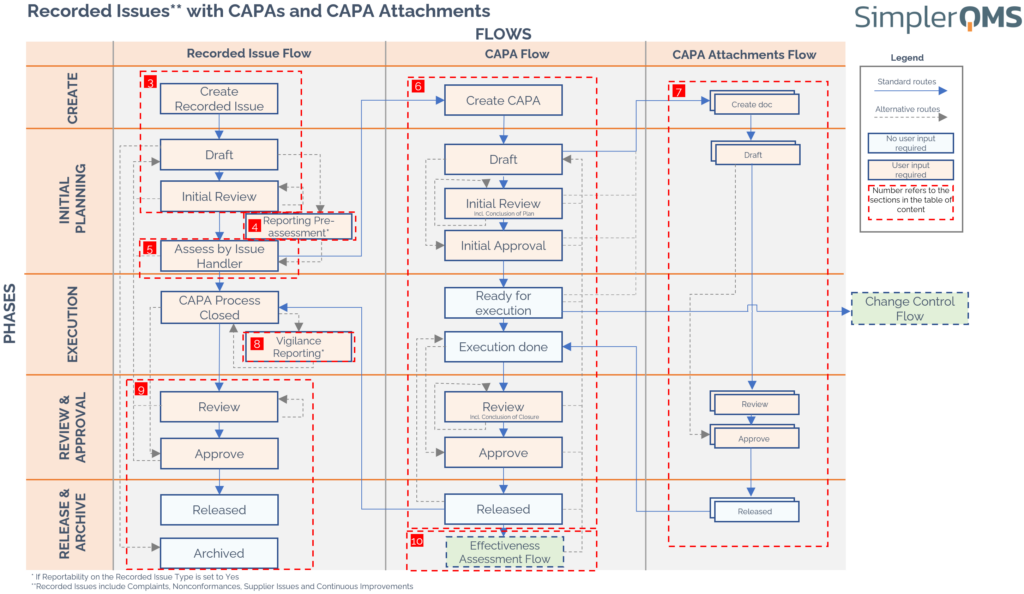
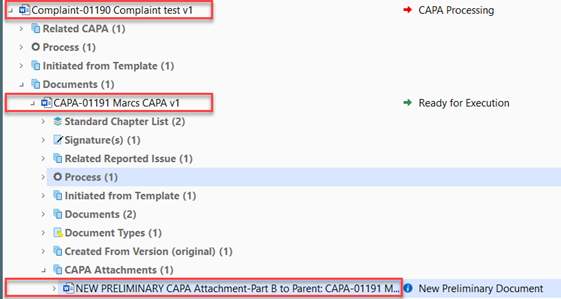
The flowchart outlines the Recorded Issue (RI), CAPA and CAPA Attachment flows, which are all divided into phases.
Section 3: Create Recorded Issue
Step 3.1 – Create Recorded Issue
Option 1: Create a Recorded Issue (stand-alone)
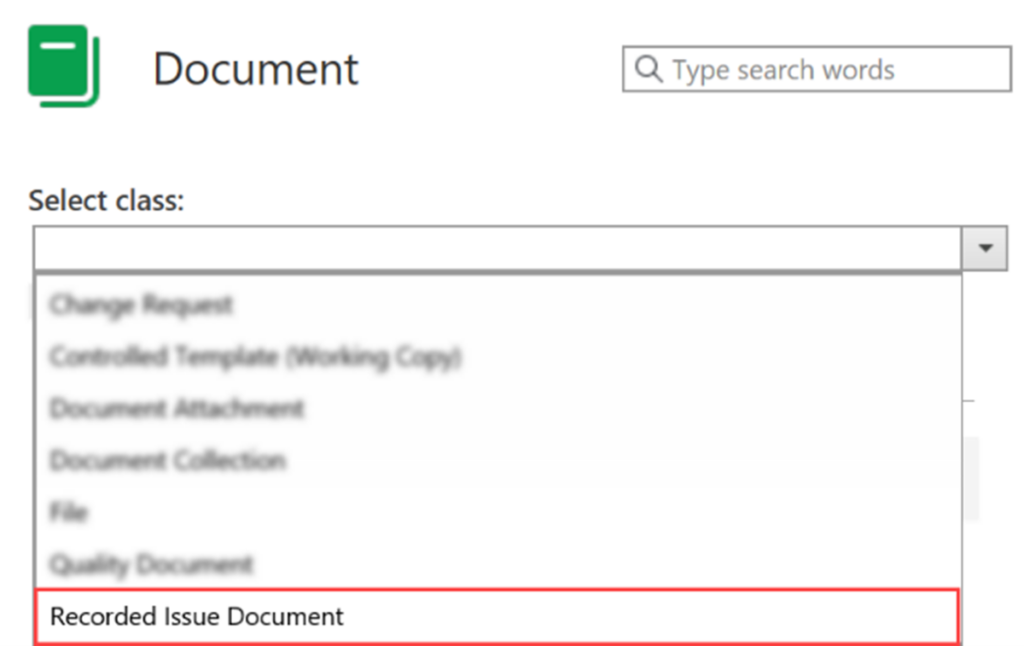
I. Click on Create (+) and choose Document from the dropdown list
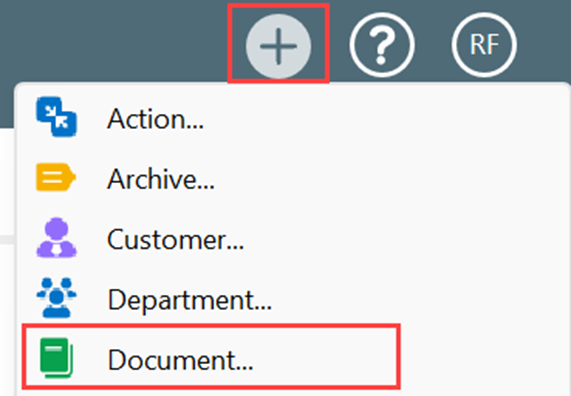
II. To create a Recorded Issue from this selection, you need Select Class as Recorded Issue Document
III. Select the approved template for Recorded Issue.
A Metadata card will pop up. (Prefilled data is based on the selected template)
Option 2: Create a Recorded Issue based on an existing record
To create a Nonconformance of a Product, select the product item. The metadata card will then be prefilled with the relevant data captured from the selected item.
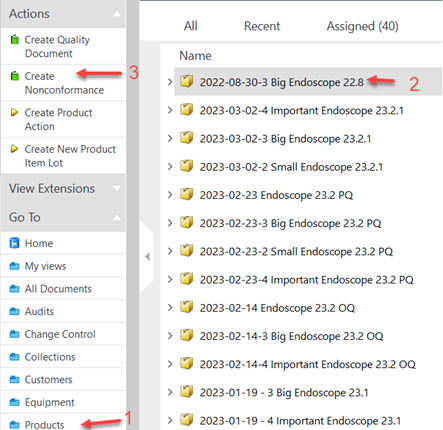
I. Under Go To, click on Products
II. Select the product item you discovered a nonconformity
III. Under Actions menu, click on Create Nonconformance
IV. Select the approved template for NC and click Next
A Metadata card will pop up.
Other recorded issues such as complaint can also be related to a customer and for supplier issues can be related to a supplier.
Step 3.2 – Fill metadata card
Part 1: Document Information
| Field name | Description | Note |
| Short Title* | The title of the Nonconformance | |
| Recorded Issue Type* | Select the Recorded Issue Type | |
| Receiving Date* (Only applicable for recorded issue type – complaint) | The date when the complaint is received. | |
| Sites* | One or more sites should be selected | |
| Document Type* | Select the relevant Document Type, often determined by the Recorded Issue Type | |
| Department* | The relevant department | |
| Process* | The relevant process | |
| Severity | Self-defined, you may create your own value list to support your description of severity | |
| Archive | If needed, select one or more archives | |
| Category | Self-defined, you may create your own value list to support your category | |
| Root Cause | Self-defined, you may create your own value list to support your root cause |
Part 2: Workflow Process Information
| Field name | Description | Note |
| Responsible Person(s)* | Select the responsible person | Can select multiple person |
| Author(s)* | Select the author (s) | |
| Initial Reviewer(s) | If needed, select the initial reviewer (s) | |
| Quality Assurance Responsible* | Assign relevant QA responsible. This will automatically assign the QA Responsible to the following roles on the CAPA (once created): Responsible Person, Initial Approvers and Approvers. | User needs to be member of the Quality Assurance Responsible user group to be selected |
| Issue Handler* | Select the person who needs to determine if CAPA is needed or not. | Member of the CAPA Handler user group |
| Reviewer(s) | If needed, select the reviewer (s) | |
| Approver(s)* | Select the approver (s) | |
| Due Date* | Select the date the RI needs to be completed (i.e., RI is Complaint base the due date on Complaint Receiving Date) | |
| Effective Date* | If you know the effective date at this stage, select the effective date | Pre-requisite upon Route for Approval |
| Latest Review Close Date | Automatically filled with the latest date the review is closed. | Read-only |
Part 3: Relations
| Field name | Description | Note |
| Related CAPA | Will automatically be filled when a CAPA is created and related to the complaint | |
| Result of Audit: | If needed, link to a relevant audit | |
| Relations | Select the relevant Relations (i.e., objects like products, customers, suppliers etc) | |
| Product* | Select the Product Item only if NC is created through Sec. 1: option 1. This is pre-filled if NC is created through Sec. 1: Option 2. | |
| Product Item* | Select one or more products that are related to the Recorded Issue | Depending on Relations |
| Product Lot List | If needed, select one or more product lots | Depending on Relations |
| Product Item Lot Number | Insert a Lot Number if needed | Depending on Relations |
| Customer | Add relations if needed for the complaint | |
| Supplier | Add relations if needed for the supplier issue | |
| Standard Chapter | If needed, link the issue to a chapter in your regulatory requirements |
I. When you have filled in the information, click Create.
II. Your Recorded Issue form will now open in Word.
Step 3.3 – Complete Draft – Author
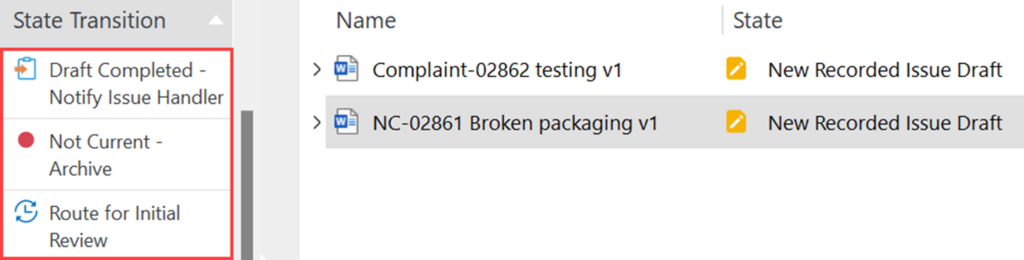
a. Draft Completed – Notify Issue Handler – this state should be clicked if the Recorded Issue is ready for CAPA Assessment. Proceed to Step 3.5.
b. Not Current – Archive – in the case that the recorded issue document was issued on accident or is no longer necessary, the author has the option to archive it
c. Route for Initial Review – involve more stakeholders in the RI drafting process by routing the document for an initial review before the CAPA assessment by the Issue Handler
Step 3.4 – Initial Review – Initial Reviewer
If the Recorded Issue Document is sent for initial review, the initial reviewer(s) can make modifications to the document in the same capacity as the author can.
I. Check out the Recorded issue document
II. Review the document and add changes if necessary
III. Save and close the document
IV. When the review has been completed, click on “My Initial Review Task Completed” under state transition.
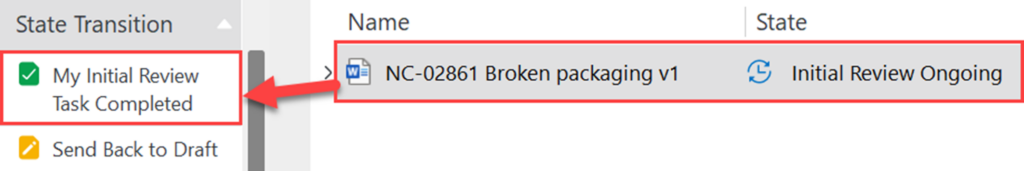
a. If there are too many modifications needed after the review process, it is recommended to route the Recorded Issue back to draft by pressing “Send Back to Draft”.
After the initial review is completed, the state changes to Initial Review Closed. In this state the author can route the Recorded Issue for another initial review if necessary.
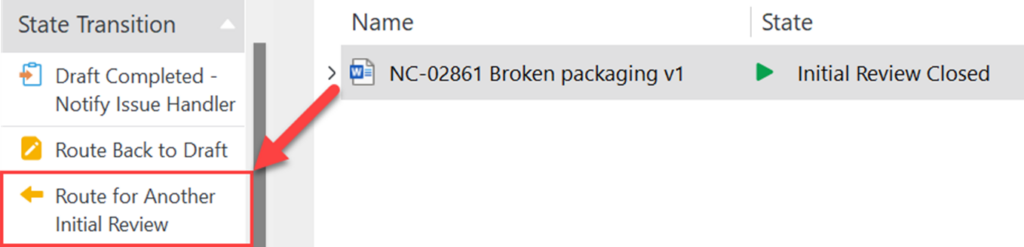
Step 3.5 – Notify the Issue Handler – Author
Once all the necessary details have been captured in the Recorded Issue Document it is ready for issue handling. The Issue Handler assesses if the recorded issue should result in a CAPA or if it can progress to final closure.
I. Complete the final details about the recorded issue.
II. Check in the RI once the drafting has been completed. Under State Transition, click on Draft Completed – Notify Issue Handler
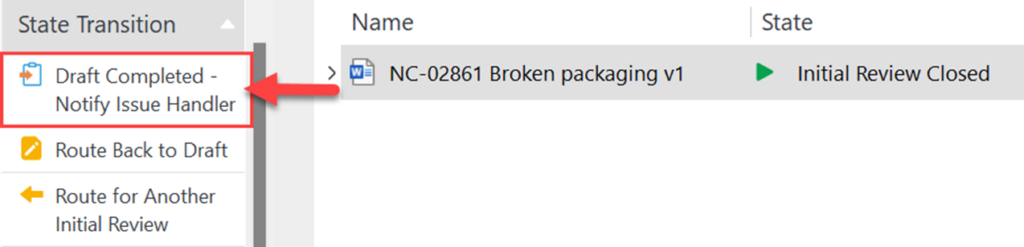
NOTE: The Responsible Person assigned to RI Document has editing access even after clicking Draft Completed – Notify Issue Handler.
Depending on the configuration of your recorded issue types (Section 1) the Recorded Issue will move into either Reporting Pre-Assessment (proceed to Section 4) or Issue Handler Assessment (proceed to Section 5).
Section 4: Reporting Pre-Assessment – Issue Handler
Based on the configuration of the selected recorded issue type, reporting becomes mandatory to determine if this specific recorded issue is reportable or not. This will be assigned to the Issue Handler to fill out the Vigilance Reporting section.
I. In the metadata of the recorded issue, fill out the Vigilance Reporting Section
Part 1: Vigilance Reporting – At Record Creation
| Field name | Description | Note |
| Is Reportable* | Determine if the recorded issue needs to be reported or not by selected Yes or No | Select Yes |
| Justification of Decision* | Enter the justification about the decision on the reporting. | |
| Reporting Details | Not mandatory at this stage, any further details of the reporting can be added into this field. Will become mandatory on Vigilance Reporting step. |
II. Click on Save
III. Click on Justification Complete under State Transition
Further details on the Vigilance Reporting will be completed once need for CAPA has been assessed and if there would be changes on the reporting details. Refer to Section 8.
State now will move to Issue Handler Assessment.
NOTE: If the recorded issue needs further details from the author, the Issue handler can route it back to draft before completing the Pre-Assessment.
Section 5: CAPA Assessment
On this section, the Issue handler determines if the recorded issue needs a CAPA.
Option 1: If CAPA is not needed
I. Select the RI and click Assessed – No CAPA Needed.

II. Sign your electronic signature and proceed, reason can be added on the signature box upon signing.
NOTE 1: If CAPA is not needed, the recorded issue proceeds with the completion of Vigilance Reporting.
Proceed to Section 8 to complete vigilance reporting.
Option 2: If a CAPA is needed
Proceed to the section 6 to Create a CAPA.
Closure of Recorded Issue with open CAPA- QA Responsible
If a CAPA is created, the recorded issue goes into a CAPA Processing state and waits for the CAPA to be processed and closed before the Recorded Issue can be closed.
The Recorded Issue can be moved forward immediately without waiting for the CAPA processing, if needed.
This is NOT a recommended option, however if the standard that you comply would allow this, make sure it describes in your company procedure, and only the QA Responsible user role can see and press the button ‘Allow Closure of Recorded Issue without CAPA Closure’ when the Recorded Issue is selected.
I. Select the recorded issue, under state transition, click on Allow Closure of Recorded Issue without CAPA Closure
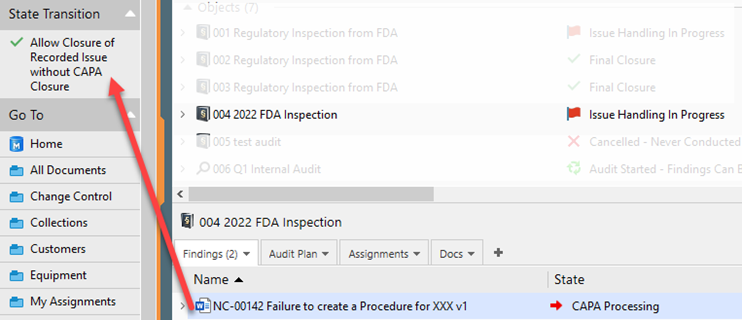
II. Enter the electronic signature to complete the closure
III. The recorded issue will then be assigned to the recorded issue author to proceed with the next step
a. Although the CAPA is still open and on Draft when the recorded issue has been closed, the recorded issue state will become CAPA Process Closed – Ready for Issue Review and Approval.
IV. The recorded issue author can now send the document either for review, approval or back to draft

NOTE: The related CAPA to this recorded issue will be able to move forward and can continue the steps as it is to complete the CAPA without affecting the state of the recorded issue.
Section 6: Creating CAPA
Step 6.1 – Create CAPA – CAPA Handler
I. Click on the Recorded Issue Document you wish to create CAPA with.
II. Select approved Template for CAPA Report and click next
III. Pre-filled metadata fields
a. Quality Assurance Responsible is copied from the NC to the CAPA’s responsible person, initial approver, and approver
b. Issue Handler is copied from NC to CAPA’s author
c. Relations are also linked automatically
Step 6.2 – Fill out metadata card for CAPA
Part 1: Document Information
| Field name | Description | Note |
| Short Title* | The title of the CAPA | |
| Sites* | One or more sites should be selected | |
| Document Type* | The relevant Document Type (i.e. CAPA) | |
| Department* | The relevant department | |
| Process* | The relevant process | |
| Archive | If needed, select one or more archives | |
| CAPA Severity | If needed, select the severity of the CAPA | |
| CAPA Status | Indication of the CAPA status (state in workflow) | Read-only |
| Category | If needed, assign one or multiple categories | |
| Root Cause | Self-define according to your own procedure | Read-only |
| CAPA Effectiveness Assessment | Will show “CAPA not assessed” until assessment is completed |
Part 2: Workflow Process Information
| Field name | Description | Note |
| Responsible Person* | Pre-filled, but can be edited | QA Responsible from the RI |
| Authors* | Select the author | |
| Initial Reviewers | If needed, select who should review initial plan | |
| Initial Approvers* | Approvers of the initial CAPA plan is pre-fille but can be edited. | QA Responsible from the RI |
| Reviewers | If needed, select the reviewers | |
| Approvers* | Approvers of the final CAPA is pre-filled but can be edited. | QA Responsible from the RI |
| Due date* | Select the due date for the CAPA | |
| Effective Date | If you know the effective date at this stage, select the effective date | |
| Latest Review Close Date | Automatically filled with the latest date the review is closed. | Read-only |
Part 3: Relations
| Field name | Description | Note |
| Related Recorded Issue | Will automatically be filled when a CAPA is created and related to the Recorded Issue | Pre-filled |
| Relations | Select the relevant Relations (i.e., objects like products, customers, suppliers etc) | |
| Customer* | Select the relevant customer. May be pre-filled, i.e., if a Complaint is created based on a customer | Depending on Relations |
| Product Item* | Select one or more products that are related to the Recorded Issue | Depending on Relations |
| Product Item Lot Number | Insert a Lot Number if needed | Depending on Relations |
| Customer | Add relations if needed for the complaint | |
| Supplier | Add relations if needed for the supplier issue | |
| Standard Chapter | If needed, link the issue to a chapter in your regulatory requirements | Pre-filled |
| Initiated from Template | Automatically filled with the latest date the review is closed. | Pre-filled |
| CAPA Attachments | Automatic once created | Short title will refer to the Main CAPA |
| Related Actions | Automatically captures all related actions to the selected CAPA |
Step 6.3 – Drafting the CAPA
CAPA is now assigned to the Author and the Issue Handler for filling out the Form and relevant section (depends on how to design your own CAPA form that will capture information needed to address the recorded issue.)
Once done with the document, fill out the Metadata CAPA Conclusions section.
CAPA Conclusions
| Field name | Description | Note |
| Initial Conclusion | Initial conclusion of the CAPA plan | Mandatory to provide prior to Initial Approval |
| Conclusion of Closure | Conclusion of Closure of the CAPA should be filled before the final approval, hence this should not be done, but in Review & Approval of the CAPA | Mandatory to provide prior to the final approval |
Current state of the Recorded Issue Document will now move to “CAPA Processing” from “Issue Handler Assessment” while the created CAPA is on a state of “New Draft”
At this stage, the author or the issue handler have an option on their Action Menu to Create CAPA Attachment
Step 6.4 – Routing for Initial Review and Approval – Author
I. The CAPA Author can have State Transition options:
a. Not Current Archive – if no longer needed
b. Route for Initial Approval without Review
c. Route for Initial Review
If there is a need to split the CAPA up into several parts (referred to as “CAPA attachments” in SimplerQMS, see Workflow Overview and for step by step instructions, go to Section 7 or to the WI for Managing Audits), these attachments can be created, drafted and reviewed/approved when this main CAPA’s initial review and/or approval has been done.
When the drafting is done and document checked-in again follow the next steps to route for initial review and/or approval or start creating the CAPA Attachments.
II. Select the CAPA either in the Detail Listings view after the Complaint is selected or find the CAPA by going to the CAPA view in the “Go To” pane.
III. The Effective date and Initial Conclusion must be entered before the document is routed for approval.
IV. Then click Route for Initial Review or Route for Initial Approval without Review.
a. If the CAPA is no longer needed, then click Not Current – Archive.
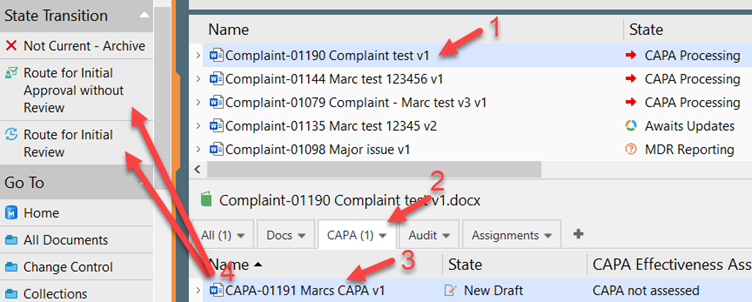
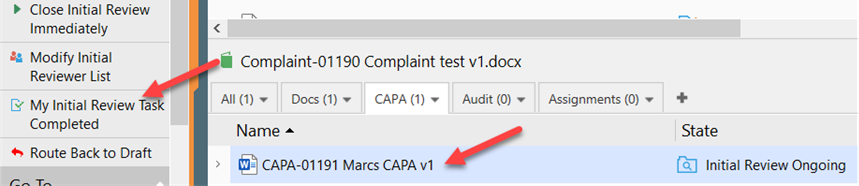
V. If CAPA has been routed for initial review, it will now be awaiting all the initial reviewers. Each reviewer must click “My Initial Review Task Completed” to move it forward for Initial Approval.
a. Alternatively, the CAPA Handler (or Process Manager) can click “Close Initial Review Immediately” and sign with electronic signature to move it directly for Initial Approval.
VI. Fill the Initial Conclusion field on the metadata card and save it before clicking Route for Initial Approval.
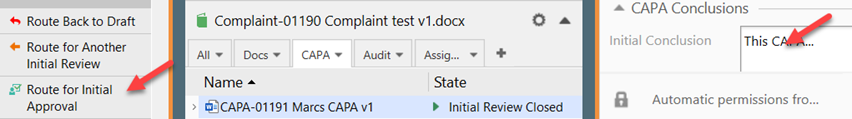
VII. Click Approve Initial CAPA and sign it, to send it for further processing (next state being Ready for Execution per flowchart in Section 1) or Reject Initial CAPA to send it back to draft. All changes made in the review process will be kept if it is rejected.
The CAPA will then transit into a state, Ready for Execution, and CAPA Attachments should be created and processed before moving on with the main CAPA, if needed. Please go to section 5, if needed, otherwise continue with Step 5.3.
Step 6.5 – Review & Approval of the CAPA
The CAPA can now be reviewed and approved. The review is optional and when the final approval is done, the CAPA will be released and go into a pending effectiveness state and await the effectiveness assessment (WI – Assessing Effectiveness of CAPA). In addition, the Recorded Issue can be closed after its own Review and Approval in the next step.
Fill Conclusion of Closure on the metadata card, with a conclusion of the CAPA. This is a requirement before the CAPA can be approved and released.

Then finalize the Review and Approval by doing the following:
I. Select the CAPA
II. Click Route for Review or Route for Approval without Review
III. Click My Review Task Completed, if reviewed
IV. Click Approve CAPA and Sign it or reject it

Approving and signing does the following:
I. The CAPA is released and moved into the state ‘Pending Effectiveness Assessment’
II. A periodic task ‘Assessment # for CAPA xx’ will be created for the Effectiveness Assessment and assigned to the Responsible Person from the CAPA. See the task in the Details Listings view.
III. The related Recorded Issue can now be closed by reviewing and approving it. You can find it in the Recorded Issue in the Detail Listings view or navigate to the relevant Recorded Issue view from the Go To menu.
Step 6.6 – Rejecting the CAPA (if needed) – Approver
I. The CAPA is sent back to Draft, which means the full review and approval process needs to be done. All changes to the document made in the past will be kept.
Section 7: Create CAPA Attachments
A CAPA Attachment is often used for splitting the CAPA process up into several parts. It is generally easiest to work with all the related CAPA documents via the Detail Listings view, when selecting the main CAPA.
A CAPA Attachment is part of the Quality Document class, with its own Document Type. In the relations section of the metadata card, the field Parent Document, shows the name of the main CAPA the attachment is linked to. Vice versa, the field CAPA Attachments on the main CAPA indicates the CAPA Attachments it is linked to.
When the CAPA Attachment has been reviewed and/or approved, the main CAPA can be moved forward for approval per the Workflow Overview
Step 7.1 – Create a CAPA Attachment – CAPA Author
I. To create a CAPA Attachment, click Create CAPA Attachment while having selected the relevant CAPA and ensure it is in the state Ready for Execution, which it is automatically transferred to after the Initial Approval.
a. If CAPA Attachments are created, they must be fully approved (or archived) before the main CAPA can be sent for Review and Approval.
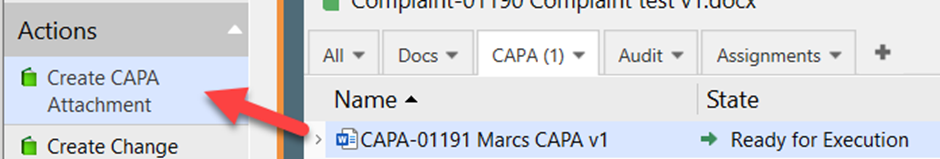
Search for the relevant template or select the class Quality Document and select a template to base the CAPA Attachment on and click Next.
Step 7.2 – Create a CAPA Attachment – CAPA Author
Section 1: Document Information
| Field name | Description | Note |
| Short Title* | A suggested CAPA Attachment name will appear, but is editable | Pre-filled |
| Sites* | One or more sites should be selected | |
| Document Type* | The selected document type is predefined by the template used but can be edited. | Depending on Relations |
| Department* | The relevant department | Depending on Relations |
| Process* | The relevant process | Depending on Relations |
| Archive | If needed, select one or more archives | Pre-filled |
Section 2: Change Control
| Field name | Description | Note |
| Requires Change Request | The document type determines if a change request is required, hence this property can only be changed by changing the doc. type. | |
| Requires Periodic Review | The document type determines if a periodic review is required, hence this property can only be changed by changing the doc type. |
Section 3: Workflow Process Information
| Field name | Description | Note |
| Responsible Person* | Add or edit the Responsible Person | Pre-filled with the QA Responsible from the RI |
| Authors* | Select the author | |
| Reviewers | If needed, select the reviewers | |
| Approvers* | Add or edit the Approvers | Pre-filled with the QA Responsible from the RI, |
| Due date | Select the due date for the CAPA Attachment | |
| Effective Date | If you know the effective date at this stage, select the effective date |
Section 4: Workflow Process Information
| Field name | Description | Note |
| Relations | Select the relevant Relations (i.e., objects like products, customers, suppliers etc) | |
| Standard Chapter | If needed, link the issue to a chapter in your regulatory requirements | Pre-filled |
| Parent Document | This is the main CAPA that the current CAPA Attachment is linked to. | Pre-filled |
| Initiated from Template | If needed, link the issue to another document | Pre-filled |
| CAPA Attachments | Not used |
I. When you have filled in the information, click Create.
II. Your CAPA Attachment form will now open in Word.
When the drafting is done and the document checked-in again, notice that the CAPA Attachment is a “child” to the main CAPA and grandchild to the Recorded Issue.
Navigate to the new CAPA Attachment by clicking CAPAs in the Go To menu and navigate to the relevant CAPA
I. Select it, choose the CAPA Attachment in Detail Listings and
II. Click Proceed to confirm the right Document Type has been entered. The Document Type, CAPA Attachment, was pre-selected when the CAPA Attachment was created, hence no action is needed to change the Document Type.
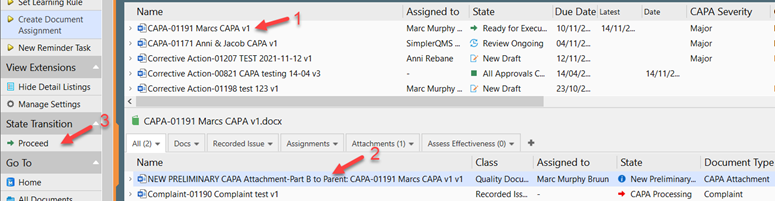
III. Route the CAPA Attachment through the Review, the reviewer checks out the document and provide comments if any. Save and close the document, back in SimplerQMS, in the state transition, click on My Review Task Completed
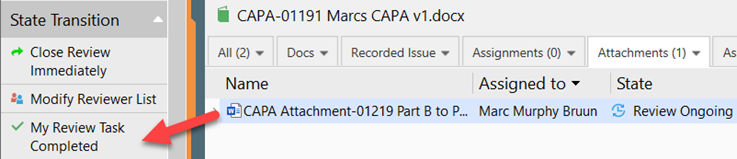
IV. When the review is completed, the author makes the final changes to the document, sets the effective date and route for approval.
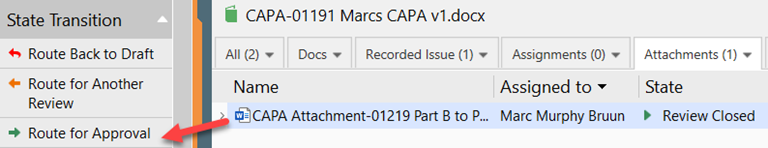
V. The approver approves the document by signing the electronic signature
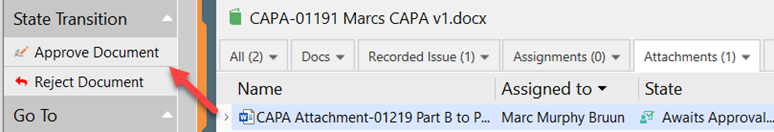
When final signature is done, the CAPA Attachment will go into a Released state and allow the main CAPA for final Review and Approval per the Workflow Overview.
Section 8: Vigilance Reporting – Issue Handler
Once the CAPA assessment has been completed, with or without CAPA, the recorded issue will proceed to the state Vigilance Reporting. (If the RI Type is set to reportable)
Fields of the Vigilance Reporting are editable to ensure that reevaluation after a more thorough CAPA investigation can be captured.
If the decision from the pre-assessment needs to be changed at this point, ensure that it is elaborated in your procedures how to follow the specific guidelines and regulations you comply with. This ensures that your vigilance reporting is done accurately and appropriately. You may want to consult with experts in your field or seek guidance from regulatory agencies to ensure that you are following the correct procedures.
I. Complete the details in Vigilance Reporting Section, and click on Save
II. In the State Transition, click on Reporting Completed
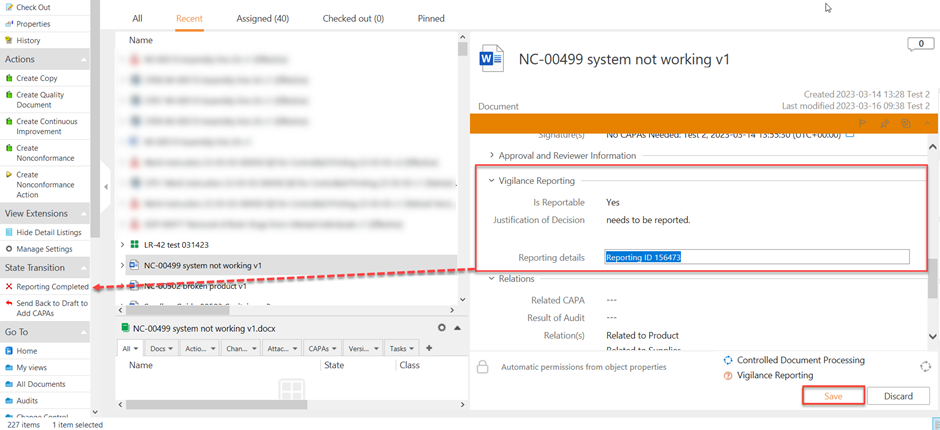
Once the reporting has been completed, the state becomes CAPA Process Closed.
NOTE: Reporting Details become mandatory if the selection on Is Reportable was Yes
Section 9: Recorded Issue: Review & Approve
This section has now been reached, based on the decision based on Section 5: CAPA Assessment:
A) The Issue Handler has assessed that no CAPA is needed
B) CAPA processing is done and the CAPA is now pending an effectiveness assessment
Step 9.1 – Send Recorded Issue for review and approval-Author
You (i.e., the complaint handler) should now process the Recorded Issue by routing it for approval with or without review (Refer to WI: Managing Quality Documents)
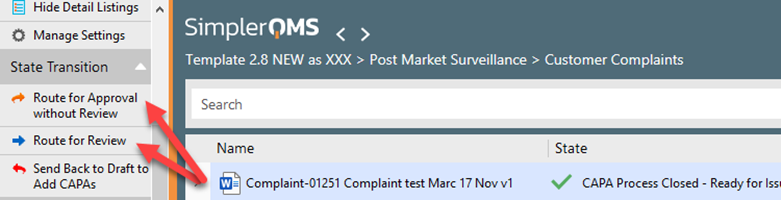
Remember that the Effective Date must be set before the document is routed for approval.
When the approval process is completed, the Recorded Issue working copy will end in the state Recorded Issue Completed and Closed. It will then be released and in a PDF version and reach its Effective state.
The CAPA has moved into the CAPA Pending Effectiveness state. To assess the CAPA effectiveness, refer to the Work instruction- Assessing Effectiveness of CAPA. If an update is necessary for the Recorded Issue, select it and under the State Transition, click on Route for Updates.
Step 9.2 – Route Recorded Issues for Updates- Author/Responsible Person
When update is necessary, the audit finding author or responsible person can accomplished the update.
I. Select the recorded issue, in the state transition click on Route for Updates

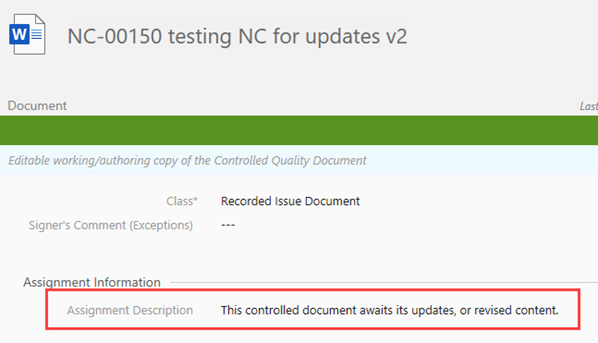
II. The recorded issue generates a version 2 of the document, and automatically assigns the document to the author. The state should be Awaits Updates

When the audit finding has been updated, it assigns to the author and will be ready for editing.
III. Author receives a notification about the recorded issue and document can be found in the author’s Assigned to Me box, drafts the document in the working copy, saves the document and check in, you can also refer to the assignment description as a guide
IV. Once the drafting is completed, the author can send the document for review or approval
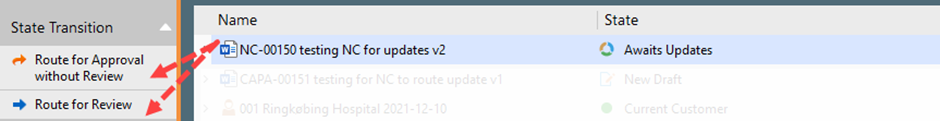
Make sure that the effective date is added before sending for approval.
V. Once all the approvals have been captured, state is Recorded Issue Completed.

Section 10: CAPA Effectiveness Assessment
Please see work instruction WI – Assessing Effectiveness of CAPA for step-by-step instruction on how to manage the Effectiveness Assessment in SimplerQMS.