CAPA Management Software for Life Sciences
Enhance your corrective and preventive actions to enforce continuous improvement and resolve issues as they arise.
TRUSTED BY
























Powerful CAPA Management Built Into an All-In-One eQMS Solution
CAPA management software by SimplerQMS helps Life Science companies to identify and resolve quality events. SimplerQMS provides a closed-loop CAPA process to help you streamline your CAPA process and achieve compliance with the requirements such as GxP, 21 CFR Part 210/211/820, ISO 13485:2016, ISO 9001:2015, ICH Q10, and more.
But that is not all. SimplerQMS’s all-in-one eQMS solution also includes and integrates other core QMS modules such as audit management, non-conformance management, risk management, training management, supplier management, complaints handling, and more.
Manage CAPA Processes With Ease
SimplerQMS CAPA Management Software facilitates data collection of complaints, non-conformances/deviations, and audit findings to ensure root cause identification takes place.
Involve your team in a CAPA plan to create an improvement-focused culture that quickly addresses quality events. Send automated notifications to ensure a specific person knows all required tasks and actions.
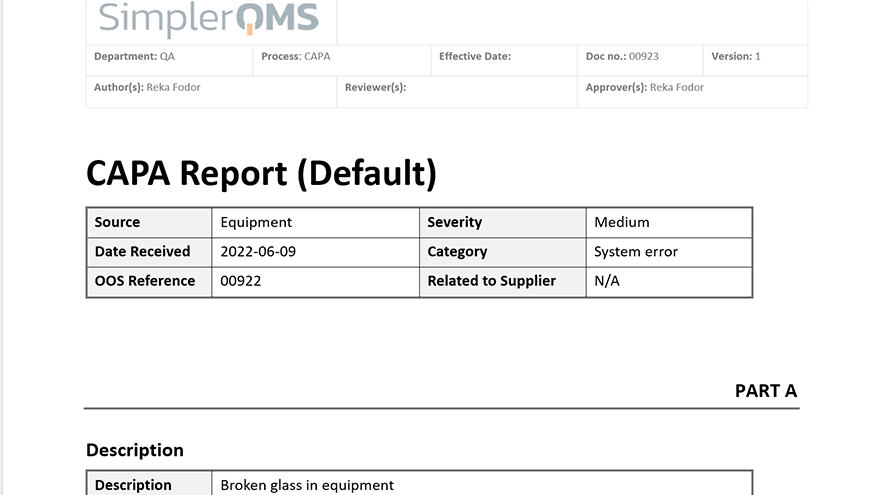
Take immediate action when quality events arise by creating new CAPA documents. You can use either your own CAPA templates or our templates which are included in SimplerQMS that the correct process is followed. Assign as many CAPAs as necessary, depending on the severity of the problem.
Gain a Clear Overview of Issues Related to CAPA
Maintain traceability of quality events using a closed-loop CAPA process in a centralized, cloud-based software solution.
Identify trends in quality with built-in dashboards and reports to interpret Key Performance Indicators (KPIs) and establish patterns. Understand how each complaint, deviation/non-conformance, or other issues might affect your organization.
Analyze CAPAs by issue type to find the most frequent problems related to products, components, customers, equipment, and suppliers. Make sure you focus your efforts on high-priority areas.
Ensure Compliance With Regulations and Standards
CAPA Software helps medical device companies meet regulatory requirements such as EU MDR and IVDR, GxP, ISO 13485:2016, 21 CFR Part 820. The solution also assists pharmaceutical, biotech, and laboratory companies in following GxP, ISO 9001:2015, 21 CFR Part 210 and 211, and ICH Q10 requirements, just to cite a few.
The system provides secure, FDA 21 CFR Part 11 compliant electronic signatures, time-stamped audit trails, and reporting capabilities.
SimplerQMS is GAMP5 validated and performs continuous re-validation every month. This way, you can be free from software validation activities. The system is compliant with ISO 13485:2016, 21 CFR Part 820, 21 CFR Part 11, and EU Annex 11 concerning electronic signatures and validation.
Take Actions Related to CAPA
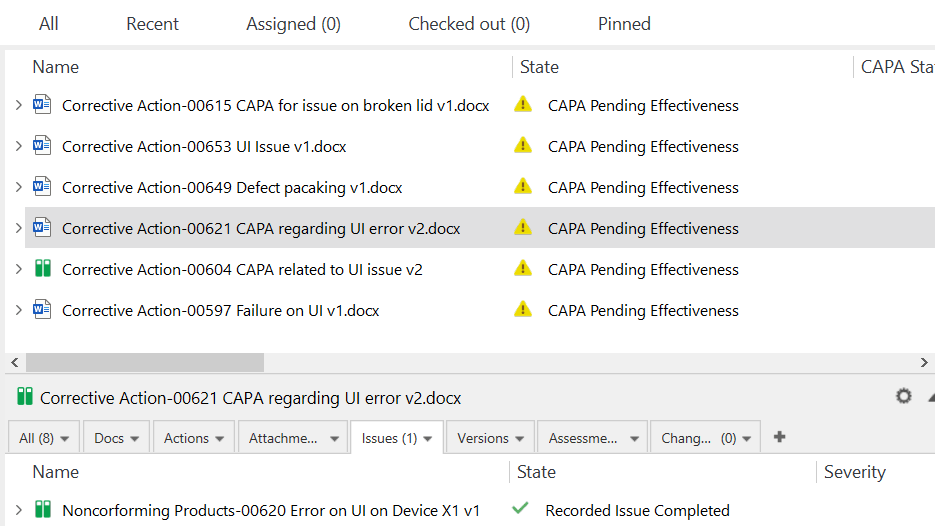
Initiate CAPA procedures directly from non-conformances/deviations, audit findings, and complaints with just a few clicks inside the CAPA management software.
Check the effectiveness of a specific CAPA by setting reminders for periodic checks to ensure necessary actions are taken on time.
Generate reports to overview CAPA status, number of CAPA open, time to resolution, overdue forms, and so on.
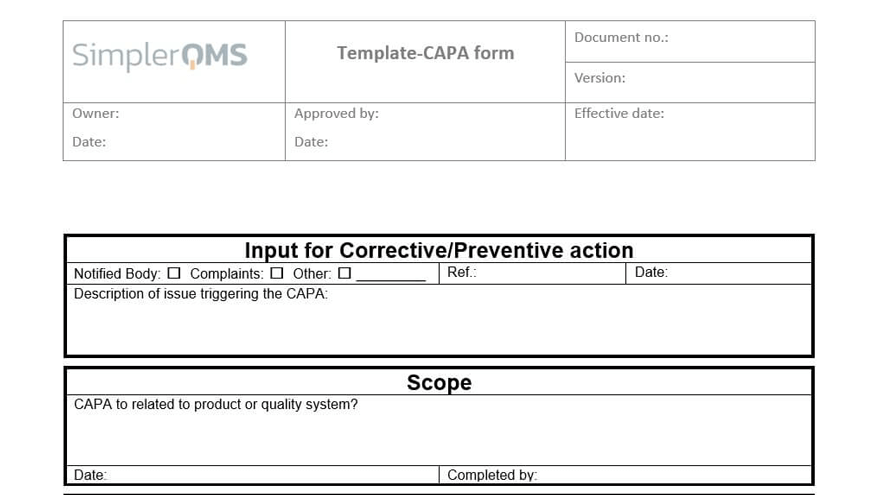
Use Form Templates for CAPA Procedures
Create new CAPA documents using templates and work in the familiar Microsoft Office interface. Create new forms and templates or migrate existing ones into the SimplerQMS solution.
SimplerQMS solution provides best-practice templates for CAPA forms and procedures which you can use as an inspiration.
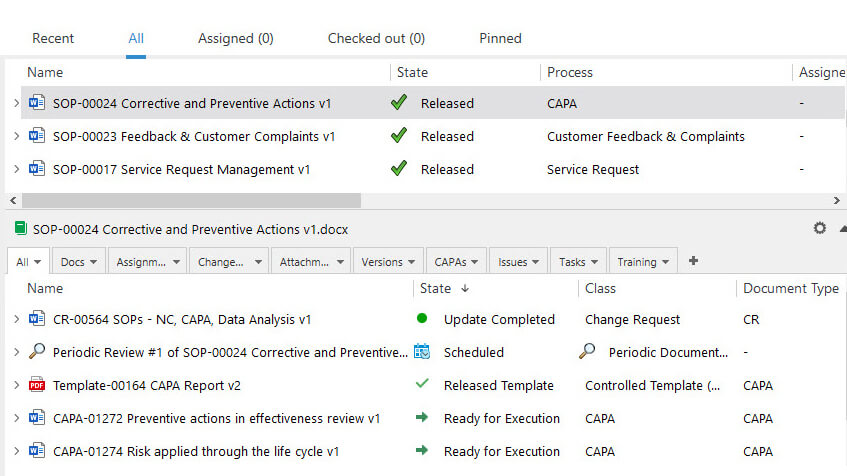
Relate documents to standards and regulation chapters using metadata for optimal references and audit trail. Complete metadata cards with ease by using an intuitive panel on the CAPA subsystem.
Monitor Your Quality-Related KPIs
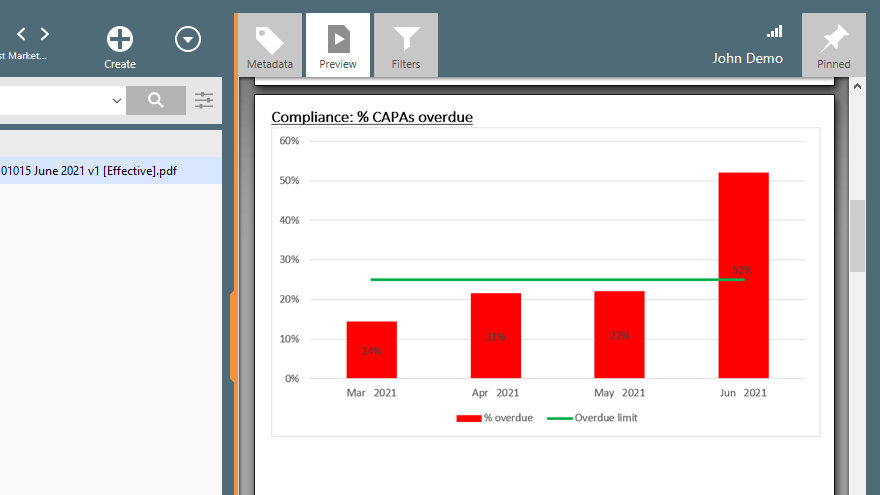
Always be aware of quality trends and closely monitor relevant quality parameters to mitigate problems and enforce continuous improvement.
SimplerQMS software provides a centralized system and closed-loop CAPA workflow so you can track areas of concern to be more proactive and avoid regulatory issues.
Monitor overdue CAPAs with KPI reports by comparing monthly results against the pre-set overdue limit.
Connect Data From Other Quality Processes
SimplerQMS CAPA solution allows you to connect information and relate documents to facilitate the retrieval of needed documentation.
Link events to other subsystems to speed up CAPA response time. Use records, files, and emails as evidence of post-market surveillance issues to support implemented actions.
With the cloud-based and closed-loop CAPA process, you have documents audit-ready all the time while maintaining traceability and making compliance easier.
What Customers Achieve With SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Much More Than Just CAPA Management
Complaint Management
Handle customers’ complaints diligently and reduce the associated risk to improve quality.
Supplier Quality Management
Manage CAPAs related to supplier activities to ensure quality throughout the entire product lifecycle.
Audit Management
Conduct, plan and manage internal and external audits with the support of integrated tools.
Risk Management
Address issues as soon as they arise to reduce risk and prevent bottlenecks.
Non-Conformance Management
Easily manage non-conformances and ensure timely resolution of quality issues.
Change Management
Be aware of all document changes and ensure your organization’s QMS complies with standards and regulations.
Frequently Asked Questions
What Is CAPA Management Software?
Corrective Action and Preventive Action (CAPA) software is a digital solution that assists Life Science companies in streamlining corrective and preventive action (CAPA) processes.
The system helps you ensure root cause identification takes place, analyze trends in quality parameters, manage CAPA related to suppliers, interlink documents, and more.
SimplerQMS provides a CAPA management solution that is a part of all-in-one QMS software. We offer broad QMS process support with document control, change management, audit management, training management, complaint handling, supplier management, risk management, and more.
What Are the Benefits of CAPA Software?
SimplerQMS CAPA software provides many benefits:
- Streamline your CAPA processes
- Identify areas of concern to take proactive actions
- Ensure compliance with regulations and standards
- Use built-in form templates for CAPA procedures
- Monitor your quality-related KPIs
- Connect data from other quality processes to retrieve documents during audits quickly
- Initiate CAPAs directly from non-conformances/deviations, complaints, and audit findings
Are There CAPA-Related Document Templates Available?
SimplerQMS provides forms and templates inside the platform to support you in achieving compliance with the Life Science industry requirements.
Our Microsoft Office integration allows you to keep working with your documents inside the familiar Word, Excel, and PowerPoint applications. In addition, you can create your own templates or just migrate existing ones.
Can CAPA Software Facilitate Audits?
Yes! Using the SimplerQMS solution, you can easily retrieve documents related to CAPA and have time-stamped audit trails for every document change.
You will have access to the document history of every product. The system records all information that is edited in the document and includes the date and time of the change, by which employee it was made, and the reason why.
How Much Does CAPA Management Software Cost?
CAPA management solution is part of a comprehensive eQMS platform that includes all core quality management system modules. We provide a complete solution from hosting, validation, and user training to ongoing support all in one subscription price.
The total investment may vary depending on the number of users you plan to have. Visit our pricing page and learn more.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.