A medical device technical file, also known as technical documentation, is a comprehensive collection of documents that contains all the technical information about a medical device.
A well-documented technical file indicates that the medical device complies with applicable regulatory requirements, such as the European Medical Device Regulation (MDR).
A compliant medical device technical file is essential for obtaining and maintaining market authorization for the device in the European Union. The medical device technical file is a requirement for approval before placing devices on the market, except for custom-made devices.
This article covers what the medical device technical file is, its importance when it is required, applicable requirements, content, examples, and how it is reviewed. We also discuss the role of QMS software in managing the medical device’s technical documentation.
Medical device companies are increasingly adopting Quality Management System (QMS) software, utilizing this solution to facilitate efficient compilation and management of the medical device technical file.
SimplerQMS provides fully validated eQMS software tailored to the needs of medical device companies. Book a demo with one of our specialists to see how it can streamline your quality management processes.
This article covers the following topics in more detail:
- What Is a Medical Device Technical File?
- Why Is Medical Device Technical Documentation Important?
- When Is a Technical File Required?
- What Are the Requirements for Medical Device Technical Documentation?
- What Is the Content of a Medical Device Technical File?
- What Is the Medical Device Technical File Format?
- What Is an Example of a Medical Device Technical File Structure?
- How Is a Medical Device Technical File Reviewed?
- What Is the Role of QMS Software in Managing Medical Device Technical Documentation?
What Is a Medical Device Technical File?
A medical device technical file is a comprehensive collection of documents that contains all the technical information and data related to a medical device. The medical device technical file includes information on the device, such as design, manufacturing, testing, clinical evaluation, and risk management.
A medical device technical file is used to demonstrate to regulatory authorities that a medical device complies with all applicable regulatory requirements.
Medical device technical file can also be referred to as medical device technical documentation.
The terms technical file and technical documentation are used in European Union (EU) requirements, such as the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).
In the United States (US), a similar document is referred to as the Device Master Record (DMR) in regulation FDA 21 CFR Part 820.
In the US, as per FDA 21 CFR Part 820 regulation, all documents related to a medical device are comprised of the Device Master Record (DMR), Device History Record (DHR), and Design History File (DHF).
Despite their different applications in different markets, the DHR, DHF, DMR, and Technical Files or Technical Documentation are all essential documents for medical device regulatory compliance and to demonstrate the device’s safety and effectiveness.
Why Is Medical Device Technical Documentation Important?
Medical device technical documentation is important because it helps demonstrate that the medical device is safe and effective for its intended use.
By meticulously documenting every aspect of a medical device, manufacturers can demonstrate to regulatory authorities that the device meets all necessary safety and performance requirements.
Furthermore, technical documentation is a requirement for conformity assessments and approval processes.
A notified body must approve medical devices before they can be marketed and sold in the European Economic Area (EEA) unless they are for research purposes or Class I non-sterile, non-reusable surgical instruments, and without measuring function devices. The approval process requires the submission of a medical device technical file.
In case a medical device malfunctions or causes harm, the technical documentation also helps find and fix the problems, protecting public health and keeping the manufacturer’s reputation intact.
Compiling the vast amount of technical documentation needed for a medical device technical file is a complex and challenging process.
SimplerQMS simplifies managing and organizing the extensive technical documentation required for medical devices, including product design and development records, clinical evaluation reports, risk management files, biocompatibility studies, validation and verification reports, and more.
When Is a Technical File Required?
A medical device technical file is required for medical devices that are marketed and sold in the European Economic Area (EEA).
Before a medical device can be legally sold, its technical file must be prepared and submitted as part of the conformity assessment process. The file is essential for demonstrating compliance with relevant regulatory requirements, such as the European Medical Device Regulation (MDR).
Any substantial changes to the medical device, its intended use, or its manufacturing process may require updating and resubmitting the technical file.
However, the requirement for a technical file may vary depending on the EU classification of medical devices.
Devices in Class I that are non-sterile, non-reusable surgical instruments and without measuring functions are not required to present the technical file to notified bodies. Due to their low risk, these devices can issue a self-declaration of conformity with EU regulations.
Still, some manufacturers choose to maintain a medical device technical file, as it helps add value, reduce risk, and demonstrate compliance.
What Are the Requirements for Medical Device Technical Documentation?
Requirements applicable to medical device technical documentation are the following:
- Medical Device Regulation (MDR)
- In Vitro Diagnostic Regulation (IVDR)
- ISO 13485:2016
NOTE
This section will mention some requirements applicable to medical device technical documentation. However, this is not an exhaustive list and more requirements might apply to companies.
Medical Device Regulation (MDR)
The Regulation 2017/745, also known as the Medical Device Regulation (MDR), is the current regulation governing medical devices in the European Union. The MDR came into effect in May 2021 and replaced the Medical Device Directive (MDD).
The MDR specifies the requirements for medical device technical documentation in Annex II and Annex III.
Annex II outlines a comprehensive list of all the information manufacturers must include in their technical documentation to demonstrate that their devices are safe and effective.
Some of the MDR Annex II technical documentation elements include:
- Device description and specification, including variants and accessories
- The basic unique device identifier (UDI) code
- Principles of operation of the device and its mode of action
- Detailed information regarding test design, study protocols, and methods of data analysis
- Label and packaging information
- Instructions for use in the languages where the device is going to be sold
- Design and manufacturing information
- Identification of all sites, including suppliers and sub-contractors
- General safety and performance information
- Benefit-risk analysis and risk management
- The risk class of the device and the justification for the classification rule
- Product verification and validation
- Software verification and validation
- The clinical evaluation plan and clinical evaluation report
Annex III specifies the requirements for technical documentation on post-market surveillance.
The manufacturer must have a proactive and systematic approach to collecting information for developing comprehensive technical documentation in the form of a post-market surveillance plan.
The post-market surveillance plan must address the collection and utilization of information concerning non-serious incidents, serious incidents, and undesirable side effects. It includes feedback and complaints provided by users, information from trend reporting, and relevant specialist or technical literature.
To ensure compliance with EU MDR, manufacturers must implement an MDR-compliant Quality Management System (QMS) that meets the specific requirements outlined in the regulation.
In Vitro Diagnostic Regulation (IVDR)
The Regulation 2017/746, better known as In Vitro Diagnostic Regulation (IVDR), currently governs the commercialization of medical devices for in vitro diagnostics in the European Union.
The EU IVDR came into effect in May 2022 and replaced the In Vitro Diagnostic Directive (IVDD). The IVDR is a more comprehensive and stringent regulation designed to improve the safety and performance of in vitro devices placed on the EU market.
The IVDR Annex II and Annex III specify the requirements for devices’ technical documentation.
Similar to MDR, Annex II provides a list of all information to be included in the technical documentation.
Examples of the IVDR Annex II elements that constitute the technical documentation include:
- The product’s name, a general description of its function, and intended users
- The unique device identifier (UDI) code
- The intended patient population and medical conditions to be diagnosed, treated, or monitored
- The device label and packaging in the languages where the device is going to be sold
- The rationale for classifying the product as a medical device
- Information to allow the design stages applied to the device to be understood
- Description of the accessories for a device
- The device’s risk class and the justification for that classification
- The general safety and performance requirements that apply to the device
- The benefit-risk analysis
- Details of any software used with the device
Annex III also outlines the requirements for technical documentation on post-market surveillance.
The manufacturer must have a post-market surveillance system integral to the quality management system.
The data gathered by the post-market surveillance system should be used to update the benefit-risk determination, clinical evaluation, safety and clinical performance, and design and manufacturing information.
ISO 13485:2016
The ISO 13485:2016 is the international regulatory standard that establishes the requirements for the medical device quality management system.
In Section 4.2.3, regarding the medical device file, the standard requires each medical device to have a technical file demonstrating compliance with all the applicable regulatory requirements.
The medical device file must include, but is not limited to, the following information:
- A general description of the medical device, its intended use and purpose, labeling, and instructions for use.
- Specifications for the product.
- Specifications or procedures for manufacturing, packaging, storage, handling, and distribution.
- Procedures for measuring and monitoring the quality of the medical device.
- Requirements for installation and servicing (as applicable).
To meet the technical file requirement and all other ISO 13485:2016 requirements, medical device manufacturers must adopt an ISO 13485:2016 compliant QMS. Such a QMS facilitates the comprehensive management and control of the entire lifecycle of medical devices, encompassing design, development, production, and even post-market activities.
What Is the Content of a Medical Device Technical File?
The technical file for a medical device must contain at least the following sections based on the EU MDR.
- Device description and specification
- Labeling and instructions for use
- Design and manufacturing information
- General safety and performance
- Risk management documentation
- Verification and validation information
- Post-market surveillance (PMS) information
Device Description and Specification
The device description and specification in the technical file serve as a comprehensive overview of the device. This overview provides detailed information about the device’s design, components, intended use, and performance characteristics.
Below are examples of technical documents that must be included:
- Engineering drawings and schematics
- Material specifications
- Manufacturing process descriptions
- Functional test plans and results
- Risk management documentation
The technical file must also include a Unique Device Identification (UDI) number for each medical device.
A Unique Device Identification (UDI) number is a globally recognized code that uniquely identifies a medical device. It consists of a numeric or alphanumeric code that identifies the device, expiration date, lot number, and serial number.
Labeling and Instructions for Use
The labeling and instructions for use section of the medical device technical file provides comprehensive guidance on the safe and effective use of the device.
These documents serve as a communication tool between the manufacturer and the device users, ensuring that users have the necessary information to operate the device correctly and minimize the risk of adverse events.
Labeling and instructions for use documents include:
- Label artwork
- Translated instructions for use
- User training materials
- Video demonstrations
The complete set of device labels and instructions for use should be included in all the official languages of the target markets.
Design and Manufacturing Information
The design and manufacturing information section of the medical device technical file provides detailed insights into the device’s design and production processes. This section includes information on the materials used, the manufacturing processes, and the quality control procedures in place.
This information is essential for demonstrating that the device is manufactured consistently and meets all applicable safety and quality standards.
Some examples of design and manufacturing documents are:
- Engineering drawings and schematics
- Material specifications
- Manufacturing process flowcharts
- Quality control plan and records
- Batch records
General Safety and Performance
The general safety and performance information in the medical device technical file demonstrates that the device complies with all applicable safety and performance requirements. It provides evidence that the device is designed, manufactured, and tested to ensure patient safety, efficacy, and reliability.
Examples of general safety and performance information include:
- Risk management documentation
- Clinical evaluation reports
- Performance testing reports
- Biological evaluation reports
- Electrical safety reports
- Mechanical safety reports
These documents include a justification, validation, and verification of the solutions adopted to meet general safety and performance.
Risk Management Documentation
Risk management documentation is an essential component of the technical file for medical devices. It showcases the systematic approach to identifying, assessing, and mitigating potential hazards associated with the device’s use.
Some examples of risk management documentation are the following:
- Hazard identification and risk assessment reports
- Risk management plan
- Residual risk evaluation report
- Benefit-risk analysis report
- Risk acceptance statement
Verification and Validation Information
The verification and validation information provides comprehensive documentation demonstrating the device’s conformity with the requirements. The documentation proves that the device meets the general safety and performance requirements.
Below are examples of verification and validation documents:
- Verification plans and test results
- Validation plans, test protocols, and test results
- Traceability matrices
- Risk mitigation traceability reports
- Change management documentation
Post-Market Surveillance (PMS) Information
Post-market surveillance (PMS) is a critical component of medical device lifecycle management, ensuring that devices continue to meet safety and performance expectations throughout their lifespan.
The PMS information section of the technical file outlines the manufacturer’s plan for monitoring the device’s safety and performance after it has been placed on the market.
Examples of PMS documentation might include:
- PMS plan overview
- Data collection procedures
- Data analysis and evaluation plan
- Risk assessment and mitigation procedures
- Regulatory reporting procedures
To ensure medical device technical documentation is comprehensive, companies usually follow the Summary of Technical Documentation (STED) format.
What Is the Medical Device Technical File Format?
A medical device STED is a document that compiles the information in a medical device technical file. It was created by the Global Harmonization Task Force (GHTF), the precursor to the current International Medical Device Regulators Forum (IMDRF), to standardize medical device regulatory submissions across markets globally, assisting both manufacturers and regulatory authorities.
The STED is intended to provide a high-level overview of the device, its design, manufacturing, and quality control processes, and the evidence supporting its safety and performance.
The information provided may include, for example, abstracts, high-level summaries, or existing controlled documents, as appropriate, sufficient to communicate key relevant information and allow a reviewer to understand the subject and assess the validity of that information.
The use of the STED should reduce costs for the manufacturer and reviewer, remove trade barriers, and facilitate timely international access to medical devices.
Additionally, the IMDRF Table of Content (IMDRF ToC) is another document that provides specific guidelines for the acceptable folder structure and file formats for regulatory submissions. For example, folder naming convention, file and submission size limitations, document security, hyperlinking in PDF files, pagination, and more.
What Is an Example of a Medical Device Technical File Structure?
Below is an example of a medical device technical file folder structure in the SimplerQMS solution.
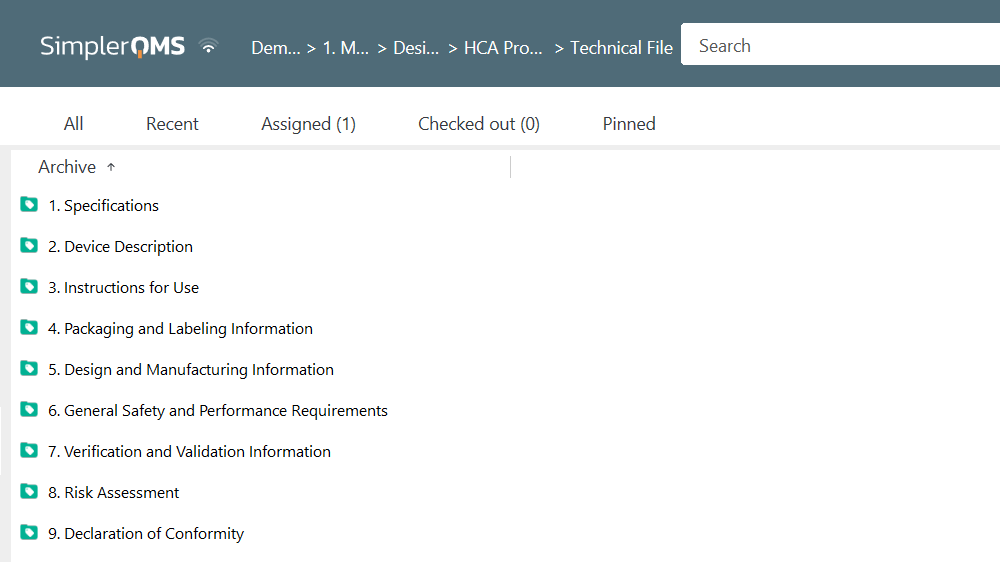
The SimplerQMS system provides a well-organized and easy-to-use platform for managing medical device technical documentation. You can create dedicated sections for device description, labeling, risk management, post-market surveillance, and more.
Note that the same documents in the SimplerQMS software can be linked to multiple archives, such as medical device technical files, DMRs, DHFs, and DHRs.
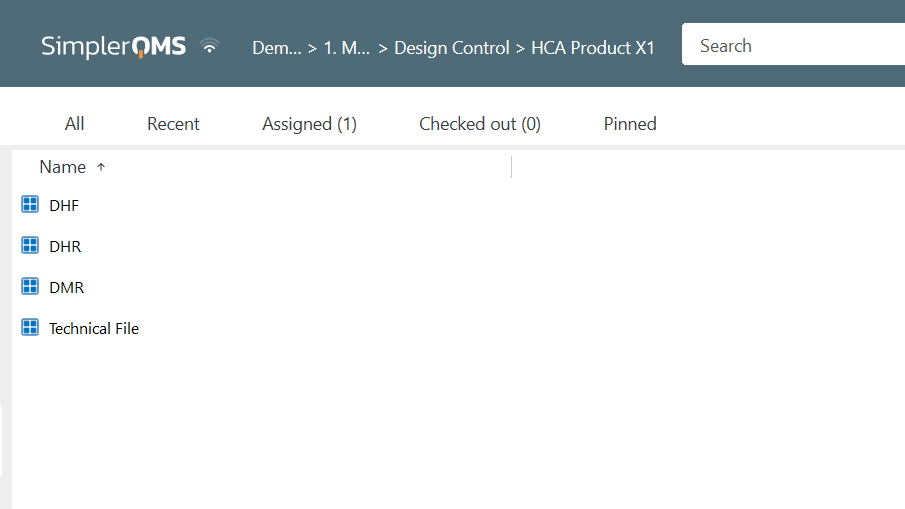
For example, a company entering the US market needs to submit various documents to comply with local regulations.
Instead of maintaining multiple copies of these documents in separate folders for each regulatory submission, the company can utilize a single electronic document that is linked to multiple archives.
When the document is updated, it is automatically reflected in all the linked archives, eliminating the need to update a document in each folder.
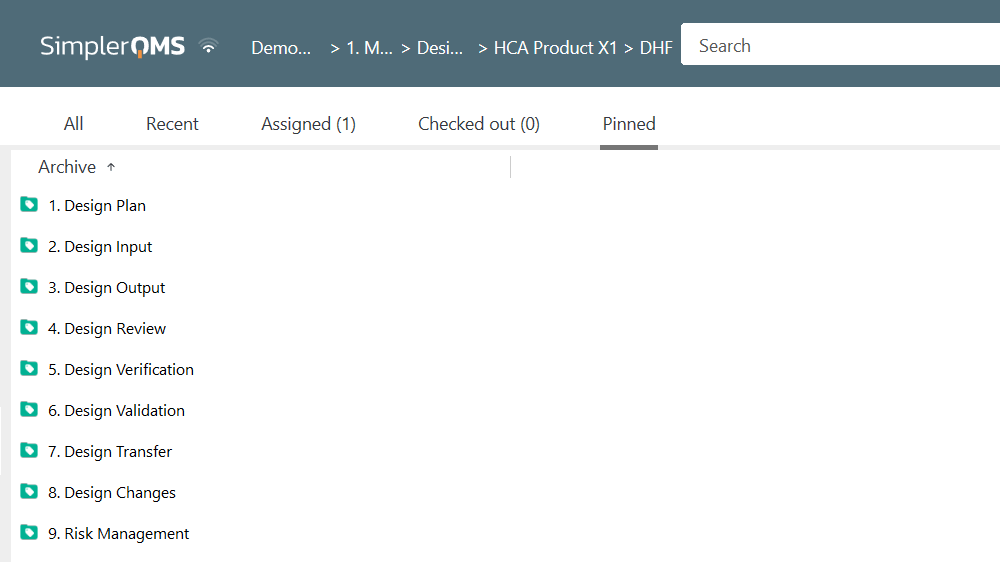
You can easily create a snapshot of each product’s current technical documentation and share it with the appropriate regulatory authorities.
Our software facilitates finding the information you need and helps ensure that your technical file is complete and compliant with all applicable regulations.
How Is a Medical Device Technical File Reviewed?
The review of a medical device technical file is a process where a regulatory authority or a notified body examines the technical documentation of a medical device. This examination aims to determine whether the device meets all applicable regulatory requirements.
The medical device technical file review is typically conducted during the medical device audit.
The audit is conducted in two stages. Stage 1 involves assessing the completeness and organization of the company’s documentation, including the technical file, usually remotely. At stage 2, the auditor evaluates the company’s processes, which take place on-site.
A QMS software platform helps medical device companies manage their technical documentation. SimplerQMS provides eQMS with a document collection tool that allows manufacturers to easily collect, organize, and store all the documents required for their technical files.
What Is the Role of QMS Software in Managing Medical Device Technical Documentation?
The role of QMS software is to streamline technical documentation management.
QMS software automates workflows for creating, reviewing, and approving technical documentation, tracks and manages versions, manages changes, and generates audit trails.
These capabilities allow medical device companies to improve the efficiency and effectiveness of their technical documentation management process, helping reduce the risk of compliance issues and improving the quality of products.
SimplerQMS provides fully validated eQMS for medical device companies to streamline quality management processes and quality documentation management, including the management of technical documentation.
Our system provides predefined workflows for tasks such as document creation, review, and approval, streamlining technical documentation management.
All technical documentation is stored in a single repository, making it easy to find and access documents when needed.
SimplerQMS system also supports compliance with the medical device industry requirements, including ISO 13485:2016, FDA 21 CFR Part 11 and 820, EU GMP Annex 11, EU MDR and IVDR, and more.
We provide comprehensive support for QMS processes, such as document control, design control, risk management, change control, training management, nonconformance management, CAPA management, customer complaint management, audit management, supplier management, and more.
Assess the benefits of investing in an eQMS solution for your company by downloading our free eQMS Business Case template.
The template will help you understand SimplerQMS’s specific benefits for your business, such as potential efficiency gains, cost savings, and compliance improvements. By presenting this information to management, you can build a strong case for implementing an eQMS.
Final Thoughts
A medical device technical file, or technical documentation, is a comprehensive document collection that contains all the technical information and data related to a medical device.
Medical device technical files demonstrate to regulatory authorities that a medical device complies with all applicable regulatory requirements.
Medical device companies are implementing quality management system (QMS) software to streamline quality management processes and manage medical device technical files and other essential documentation.
SimplerQMS is a fully validated (GAMP 5) eQMS software solution for medical device companies that streamlines quality management, including the management of technical documentation.
The system streamlines the process of creating, reviewing, and approving technical documents through predefined workflows. Document changes are automatically tracked, and new versions of the documents are recorded in time-stamped audit trails.
Experience how SimplerQMS can streamline your quality management processes and help you manage your technical documentation more efficiently by booking a demo with our Quality Solution Experts.