FDA 21 CFR Part 11 and EU GMP Annex 11 address similar concerns and aim to achieve the same goals, namely data integrity, comprehensive audit trails, limited system access, trained users, and more. However, there are notable differences between these regulations.
Understanding these differences is essential for Life Science companies operating in both the American and European markets to ensure compliance and avoid potential nonconformances.
This article will discuss the key differences between 21 CFR Part 11 and EU Annex 11. We will explain what each entails, their requirements, and their differences. We will also show how SimplerQMS can help you achieve compliance with both.
Life Science companies are increasingly adopting electronic systems to manage regulated processes. These systems provide technology to streamline and automate processes, reducing risks and improving efficiency.
These electronic systems also help companies comply with several requirements, such as FDA 21 CFR Part 11 and EU GMP Annex 11.
SimplerQMS provides eQMS software designed for Life Science companies and complies with 21 CFR Part 11 and EU GMP Annex 11. Book a demo and talk to our quality experts today to get a more comprehensive understanding of the QMS software.
This article covers the following topics in more detail:
- What Is the FDA 21 CFR Part 11?
- What Is EU GMP Annex 11?
- What Are the Key Differences Between 21 CFR Part 11 and Annex 11?
- How SimplerQMS Supports Your Compliance With 21 CFR Part 11 & EU Annex 11
What Is the FDA 21 CFR Part 11?
The 21 CFR Part 11 is a part of the Food and Drug Administration (FDA) regulation that establishes criteria for using electronic records and signatures.
The scope of 21 CFR Part 11 encompasses electronic records created, modified, maintained, archived, retrieved, or transmitted under FDA requirements.
The regulation applies to any Life Science companies that utilize electronic systems for recordkeeping and signature processes subject to FDA regulations within the United States. This includes pharmaceutical manufacturers, contract research organizations (CROs), biotechnology companies, and medical device manufacturers, among others.
Its primary purpose is to ensure the integrity, authenticity, and reliability of electronic records and signatures used in FDA-regulated activities by setting forth comprehensive requirements.
What Are the Key Requirements of the FDA 21 CFR Part 11?
FDA 21 CFR Part 11 specifies the requirements for electronic records and signatures to be considered trustworthy, reliable, and equivalent to paper records and signatures on paper.
Here, we will provide a concise overview of the sections of 21 CFR Part 11 and emphasize its key requirements. If you are interested in gaining a more thorough understanding of the various parts and specific requirements of 21 CFR Part 11, we recommend reading our comprehensive article on 21 CFR Part 11 requirements.
NOTE
Please note that the information presented here is intended for educational purposes only. Companies should always refer to the FDA 21 CFR Part 11 regulation for official data.
Electronic Records Requirements
Companies must follow specific procedures and controls to maintain the records’ authenticity, integrity, and, if needed, confidentiality.
Some of the required procedures and controls for compliant electronic records include the following:
- 21 CFR 11.10: Controls for closed systems ensure system validation, record retrieval, system access controls, audit trails, operational checks, user training, and more.
- 21 CFR 11.30: Controls for open systems include the exact requirements as closed systems, with added security measures, such as document encryption and appropriate digital signature standards.
- 21 CFR 11.50: Signature manifestations, such as the printed name of the signer, date, time, and meaning of signature, should be included in the electronic records.
- 21 CFR 11.70: Electronic signatures should be linked to their respective records to prevent falsification.
Electronic Signatures Requirements
To be valid and legally binding, electronic signatures must meet specific procedures and controls. These requirements help to ensure that the signer is who they say they are, that the signature is not tampered with, and that the document is genuine.
Here are some of the requirements for electronic signatures:
- 21 CFR 11.100: General requirements include requirements for electronic signatures to be unique and have their user identity verified.
- 21 CFR 11.200: Electronic signature components and controls specify using at least two distinct identification components, such as identification code and password, controls for sequence signing, and more.
- 21 CFR 11.300: Controls for identification codes and passwords ensure the uniqueness of combined identification codes and passwords, loss management procedures, and transaction safeguards, among other requirements.
What Is EU GMP Annex 11?
The EU GMP Annex 11 is a guideline the European Union (EU) issued regarding using computerized systems in good manufacturing practices (GMP) for human and veterinary medicinal products.
The guideline outlines requirements for implementing and validating computerized systems to ensure data integrity, accuracy, and security in GMP-related activities.
It applies to all companies operating within the European Union in the manufacture of medical products with GMP-regulated activities, including pharmaceutical, contract manufacturing organizations (CMO), and others.
The purpose of EU Annex 11 is to provide guidance for interpreting the principles and guidelines of computerized systems. It helps medicinal product manufacturers assess and implement necessary controls to minimize risks and identify vulnerabilities.
What Are the Key Requirements of EU GMP Annex 11?
Compliance with EU GMP Annex 11 is essential for companies who want to ensure that their computerized systems are used in a way that meets regulatory requirements in GMP-regulated activities.
Below, we provide a brief explanation of the key requirements of EU Annex 11. However, please note that this is not an exhaustive list. For a more comprehensive understanding of EU Annex 11, we recommend reading our dedicated article on EU Annex 11 requirements.
NOTE
Please note that the information presented here is intended for educational purposes only. Companies should always refer to the EU Annex 11 guideline for official data.
General Requirements
- Risk Management: The risks associated with computerized systems should be assessed, and companies should implement controls to mitigate those risks.
- Personnel: All personnel who use computerized systems must have the appropriate training and qualifications to perform their tasks.
- Suppliers and Service Providers: Formal agreements should be in place with third-party suppliers and service providers that clearly define their responsibilities.
Project Phase
- Validation: Companies should have a validation plan that covers the relevant steps of the lifecycle of the computerized system. The validation plan should be based on the risk assessment and justify the standards, protocols, acceptance criteria, procedures, and records used.
Operational Phase
- Data Storage: Stored data must be secure and checked for accessibility, readability, and accuracy. Access to data needs to be ensured throughout the retention period.
- Printouts: Printed copies of electronically stored data must be available and indicate if any data has been changed since the original entry.
- Audit Trails: The reason for any change or deletion of GMP-relevant data should be documented. Audit trails should be available in a comprehensible form and regularly reviewed.
- Electronic Signature: Electronic signatures need to be equivalent to their handwritten counterparts. Records must include the time and date that they were signed and have the signatures permanently linked to them.
What Are the Key Differences Between 21 CFR Part 11 and Annex 11?
21 CFR Part 11 and EU Annex 11 have different scopes and requirements. However, they both are important frameworks for ensuring the quality and integrity of data in electronic records.
It is essential to understand the differences between the two frameworks to comply with the requirements that apply to your specific needs.
Overall, 21 CFR Part 11 is more specific and detailed in its requirements than EU Annex 11. 21 CFR Part 11 outlines specific requirements that must be met, while EU Annex 11 provides general guidance to the areas of compliance.
When comparing these frameworks, several aspects are considered. These encompass the following elements.
Definition
The FDA 21 CFR Part 11 regulatory framework outlines the criteria governing electronic records and signatures. It is a comprehensive set of requirements for ensuring electronic documentation’s integrity, authenticity, and reliability within FDA-regulated industries.
On the other hand, the EU Annex 11 is a guideline that provides general principles for computerized systems used in Good Manufacturing Practice (GMP) activities.
Market
Another difference between 21 CFR Part 11 and EU Annex 11 is their geographical applicability.
21 CFR Part 11 applies to companies operating in the United States market under FDA regulations.
In contrast, EU Annex 11 applies to companies operating in the European Union market and conducting GMP activities according to EU guidelines.
Scope
The difference in the scope of the frameworks is that 21 CFR Part 11 focuses on electronic records and signatures in open and closed computer systems used in FDA-regulated activities, ensuring their integrity, authenticity, and reliability.
EU Annex 11 addresses computerized systems used in GMP-regulated activities, providing appropriate implementation and management guidelines.
While 21 CFR Part 11 specifically targets electronic records and signatures, EU Annex 11 takes a broader approach by encompassing the whole computerized system.
Regulatory Authority
Another difference lies in the regulatory authorities overseeing the frameworks.
21 CFR Part 11 is regulated by the Food and Drug Administration (FDA) in the United States. While EU Annex 11 is regulated by the European Medicines Agency (EMA) in the European Union.
Regulatory Status
There is a significant difference between the regulatory status of the frameworks.
21 CFR Part 11 is a regulation and requires mandatory compliance when utilizing electronic records and signatures. It carries legal weight and enforceability, meaning companies operating under FDA regulations must comply with the 21 CFR Part 11 requirements.
On the contrary, EU Annex 11 is a guideline that offers recommendations and guidance for interpreting the GMP principles. It is not legally binding, meaning companies operating within the European Union market are not obligated to have compliance.
Audit Trails
The framework requirements for audit trails differ on the records they apply to.
In 21 CFR Part 11, audit trails are required for all electronic records, encompassing a broader range of data.
On the other hand, in EU Annex 11, audit trails are specific for GMP-relevant data.
This difference indicates that 21 CFR Part 11 places a bigger emphasis on the comprehensive audit trail implementation. Meanwhile, EU Annex 11 narrows its focus to GMP-specific data.
Risk Management
While 21 CFR Part 11 does not explicitly mandate a risk assessment, EU Annex 11 specifies that a risk assessment should be conducted throughout the lifecycle of the computerized system.
The risk assessment in EU Annex 11 encompasses patient safety, data integrity, and product quality considerations.
The difference indicates that EU Annex 11 emphasizes the proactive identification and management of risks associated with computerized systems. However, 21 CFR Part 11 does not explicitly require a formal risk assessment process.
The infographic below illustrates these main differences between the 21 CFR Part 11 and EU Annex 11.
When it comes to managing regulated activities, many Life science companies are adopting electronic Quality Management Systems (eQMS) compliant with 21 CFR Part 11 and EU Annex 11.
Such eQMS solutions provide a comprehensive and streamlined approach to managing quality management processes while helping ensure compliance.
How SimplerQMS Supports Your Compliance With 21 CFR Part 11 & EU Annex 11
SimplerQMS offers an eQMS software solution compliant with FDA 21 CFR Part 11 and EU GMP Annex 11 requirements.
Here are some of SimplerQMS’s key capabilities that help achieve compliance with 21 CFR Part 11 and EU Annex 11.
System Validation
SimplerQMS is fully validated according to ISPE GAMP5, a risk-based approach to computerized systems.
The system is tested and continuously validated to meet 21 CFR Part 11 and EU Annex 11 requirements. We handle all system validation activities. There are no additional expenses, resources, or time commitments to software validation from our customers.
Data Storage and Retrieval
Our cloud-based system enables easy access to documents from anywhere at any time.
Records are securely stored, ensuring availability for inspection throughout the retention period. A search feature simplifies record retrieval by keyword search in document titles and content.
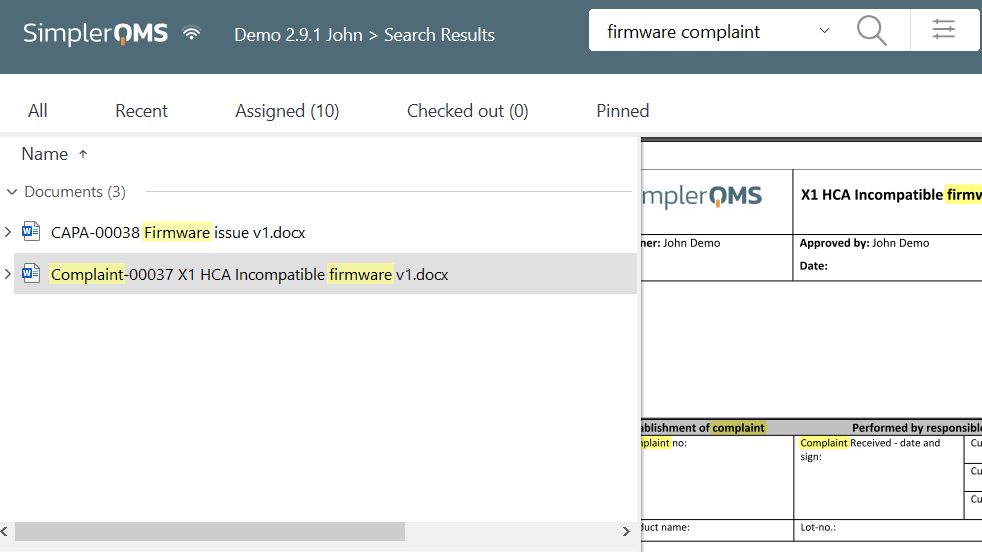
Personnel Training
We provide comprehensive training to individuals on how to use our system. We offer unlimited training sessions, workshops, written material, and videos to facilitate learning.
Our integrated Training Management module also streamlines training management in your organization by allowing the easy creation of training plans, assignments, progress monitoring, and automated notifications.
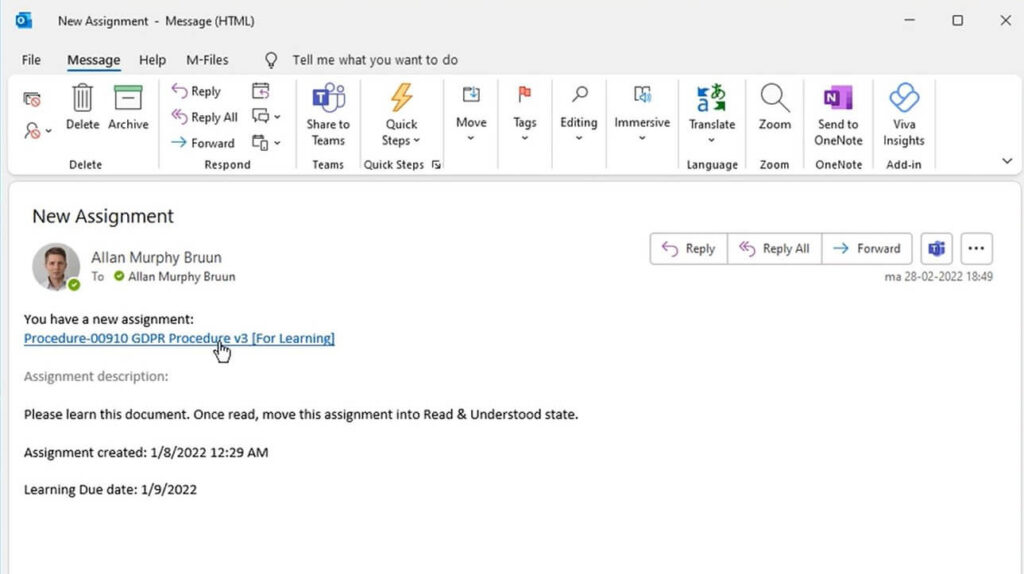
Record Generation
SimplerQMS simplifies record generation by providing document templates.
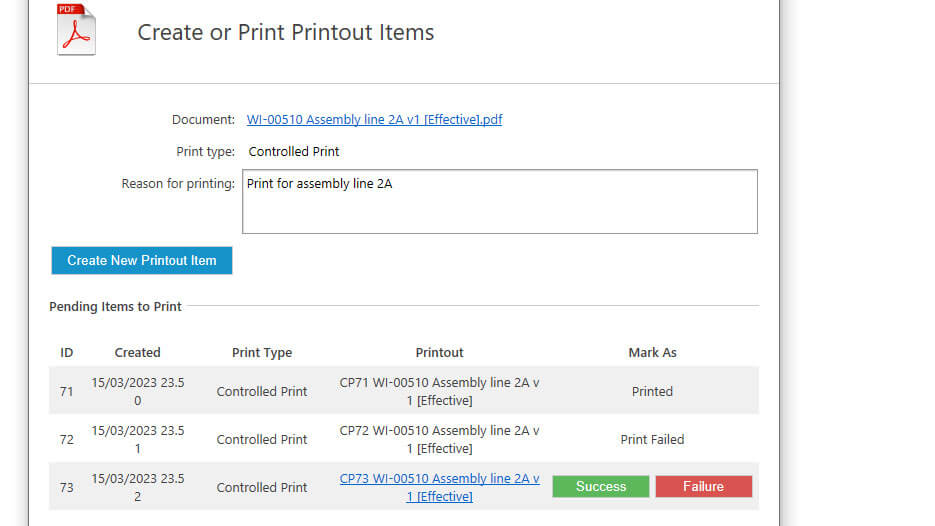
We support copying and exporting records for audits and regulatory inspection, including printable documents. Our controlled printing capability ensures tracking of printout status.
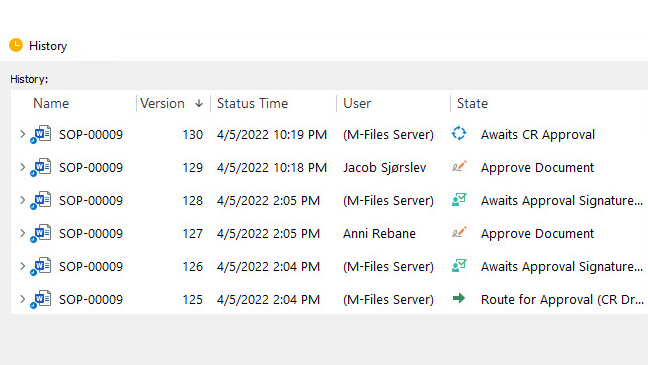
Audit Trails
SimplerQMS provides computer-generated and time-stamped audit trails, automatically storing each record version.
The audit trail is a chronological history of all actions performed to a record that includes the user, date, time, and actions taken.
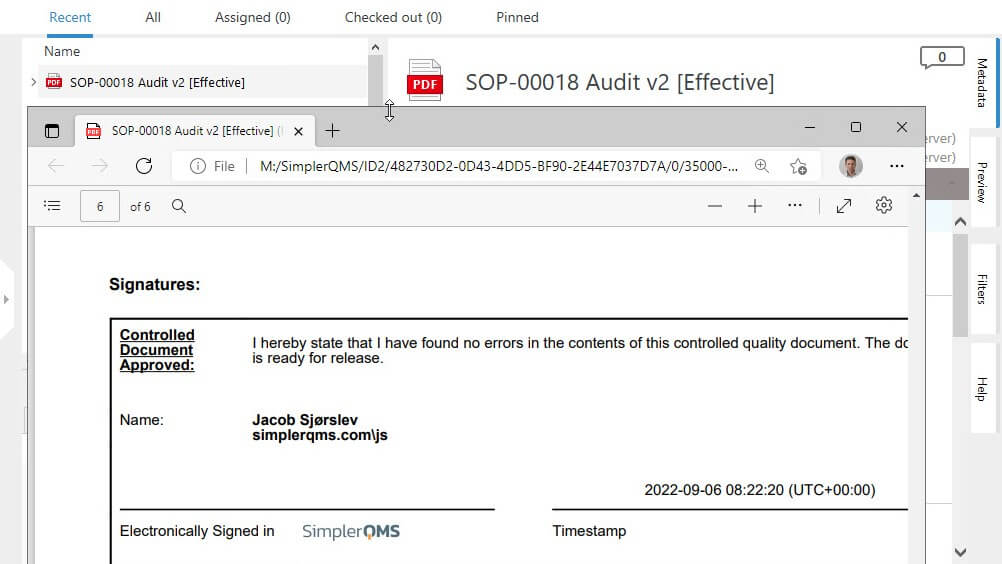
Electronic Signatures
We provide EU Annex 11 and 21 CFR Part 11 compliant electronic signature capabilities.
In SimplerQMS, electronic signing is only possible one record at a time, with all signature components, that includes the signer’s name, date, time, and meaning of the signature.
Each signing credential is unique and linked to the corresponding record, ensuring integrity and preventing falsification.
System Access Control
SimplerQMS integrates with Microsoft Entra ID (previously known as Microsoft Azure Active Directory) for secure identity and access management.
Access to the system and specific records is limited only to authorized personnel. Users are assigned unique identification codes and passwords to ensure secure access, safeguarding the confidentiality of electronic records.
SimplerQMS offers more than 21 CFR Part 11 and EU Annex 11 compliance. We help companies comply with several Life Science requirements, such as 21 CFR Part 211, 212, and 820, ISO 13485:2016, ISO 9001:2015, MDR and IVDR, EU volume 4 GMP, ICH Q10, and more.
Our comprehensive QMS software solution includes various QMS modules – document management, change control, employee training, customer complaint, CAPA management, supplier management, deviation management, and more.
If you are considering implementing an eQMS solution in your company, download our free eQMS Business Case template.
This template will help you calculate the real economic benefits and time savings of an eQMS and present your finding to management.
Final Thoughts
21 CFR Part 11 and Eu Annex 11 differ regarding their scope, applicability, and requirements.
While 21 CFR Part 11 is a US regulation governing electronic records and signatures in all FDA-regulated activities. EU Annex 11 is an EU guideline for the computerized systems in GMP-regulated activities for medicinal products.
Life Science companies are increasingly implementing electronic systems such as eQMS software to manage quality processes and improve compliance efforts.
SimplerQMS provides a tailored eQMS for Life Science companies to streamline their quality processes and help achieve compliance.
Experience firsthand how SimplerQMS can assist you in streamlining your QMS processes and achieving compliance with Life Science requirements. Schedule a personalized demo with our knowledgeable eQMS system experts today.