Product Management Solution for Life Sciences
Optimize your product management workflow. Ensure efficient handling of product documentation and streamline processes from conception to market.
TRUSTED BY
























Product Management Built Into an All-In-One eQMS
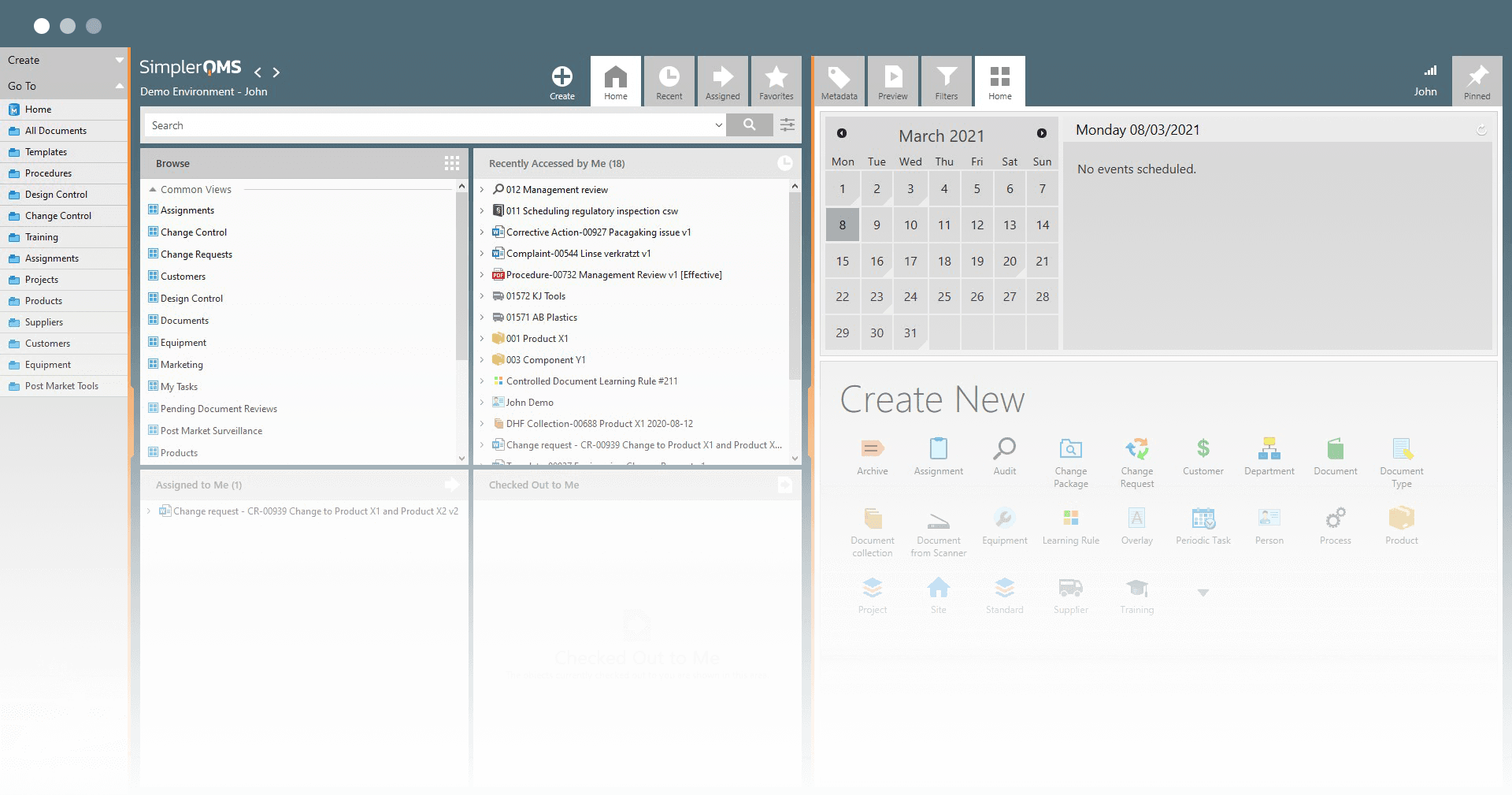
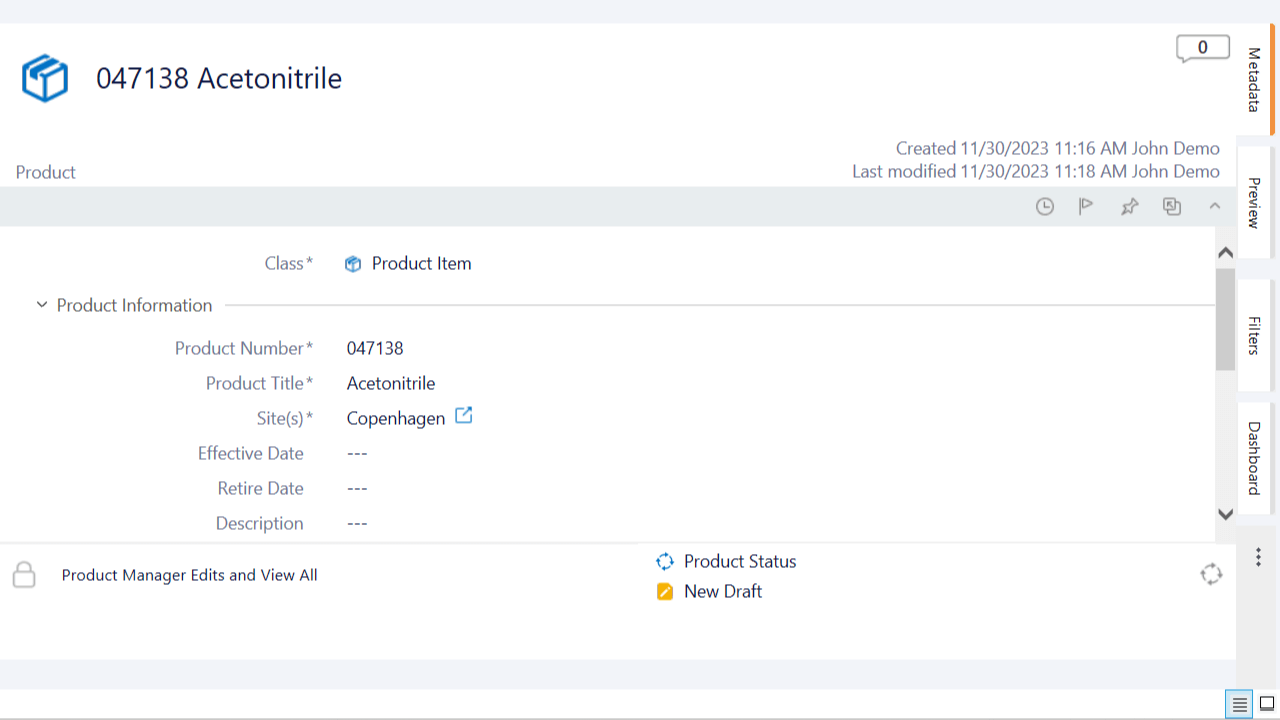
SimplerQMS provides a Product Management module within a comprehensive QMS software to help Life Science companies manage all products and product documentation in one single system.
The software provides centralized product documentation management, streamlined automated workflows, and enhanced document traceability, offering numerous benefits.
You can relate products to various items within the system, including documents, equipment, suppliers, audits, and more. Additionally, you can capture unique data about each product using custom fields.
The Product Management module is an integrated part of the SimplerQMS solution. The software encompasses all QMS modules, including document control, change management, training, CAPA management, equipment management, complaint management, and audit management, among others.
Centralize Product Information
Have a single source of truth for all product-related information, including product specifications, documents, and relations, streamlining accessibility and organizational efficiency.
Foster collaboration by enabling cross-departmental access to approved product data, and streamline communication.
Minimize errors and maintain compliance by ensuring consistent and accurate information across the organization.
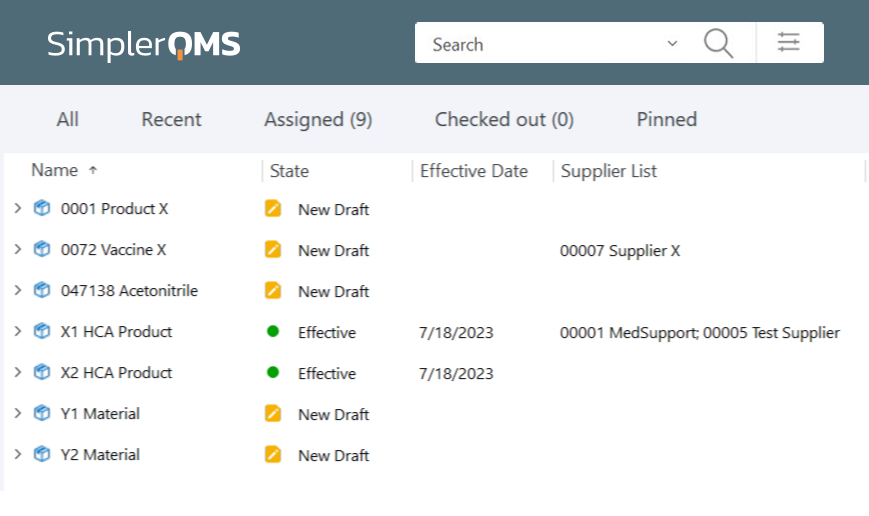
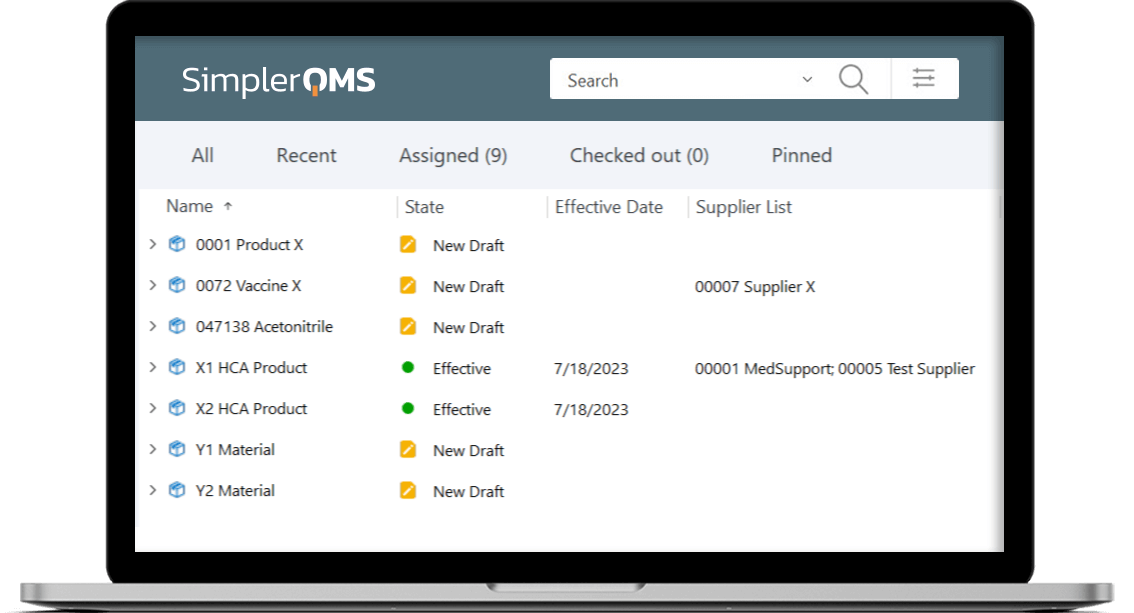
Store a High Number of Products in the System
Effortlessly scale the amount of product data needed to meet the demands of a growing product portfolio. Ensure your storage capacity evolves with your business requirements with a cloud-based system.
Store up to 150,000 documents in the system by default. Increase storage space automatically when additional user licenses are purchased.
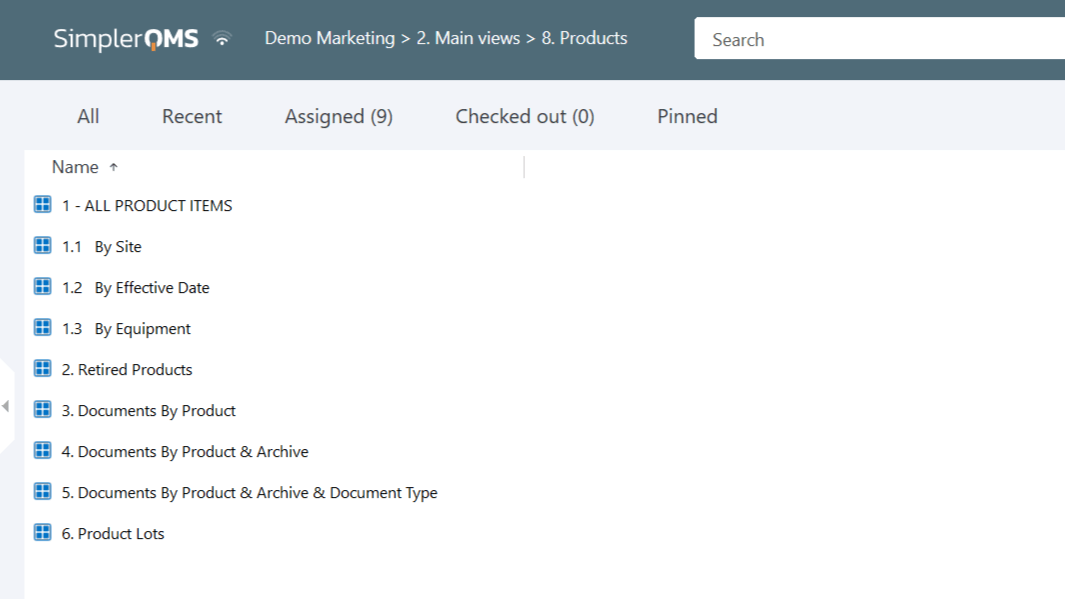
Organize products by customized views to categorize and store product documentation, streamlining workflow and enhancing accessibility.
Control Product Information With Change Control
Ensure the integrity of your products through the Change Control process. Employ change request workflows to protect product information against unauthorized modifications.
Actively ensure that no new risks are introduced to the product when making changes.
Automatically track all actions performed, documenting changes in the product history. Obtain a clear audit trail of each product’s development.
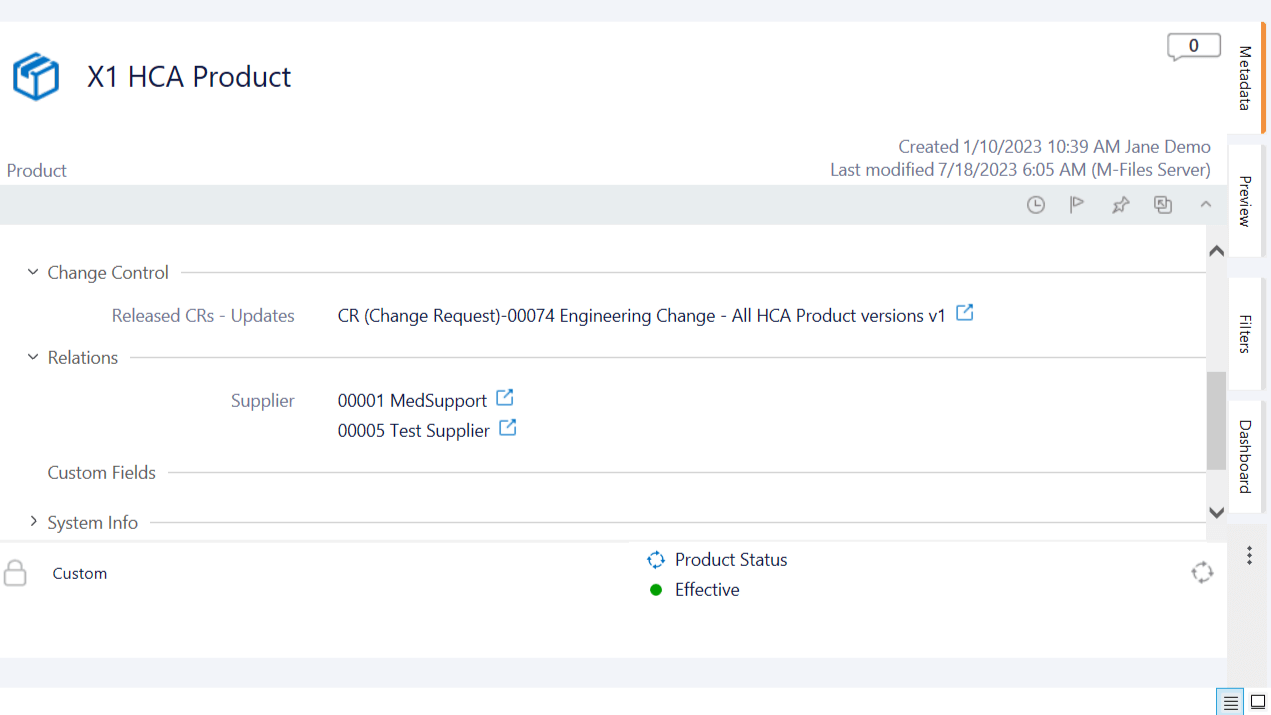
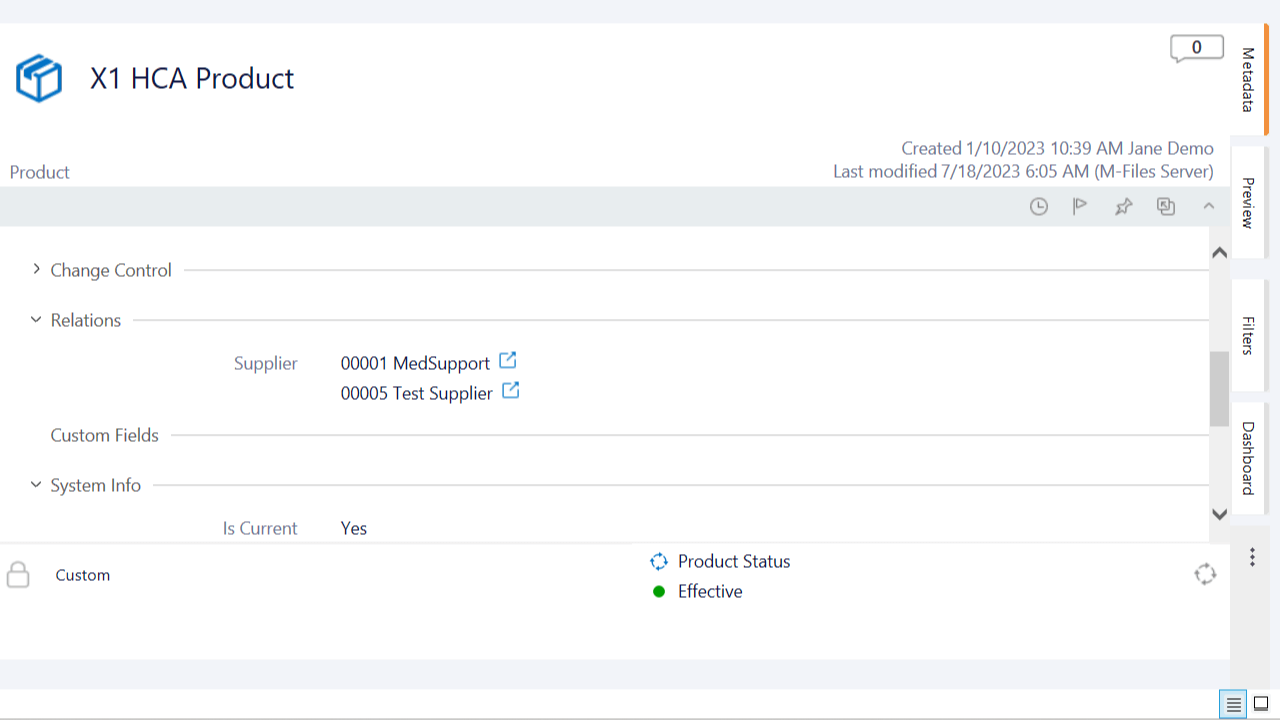
Relate All Product Data
Relate product data effortlessly within the system. Link products to documents, batch master records, product release notes, suppliers, risk assessments, CAPAs, nonconformances, deviations, and more.
Facilitate collaboration across departments by relating product information. Have documents readily accessible and improve cross-functional communication.
Simplify regulatory submissions with linked product data to facilitate the compiling and organization of documents. Create document collections to simplify sending documents for review and approval.
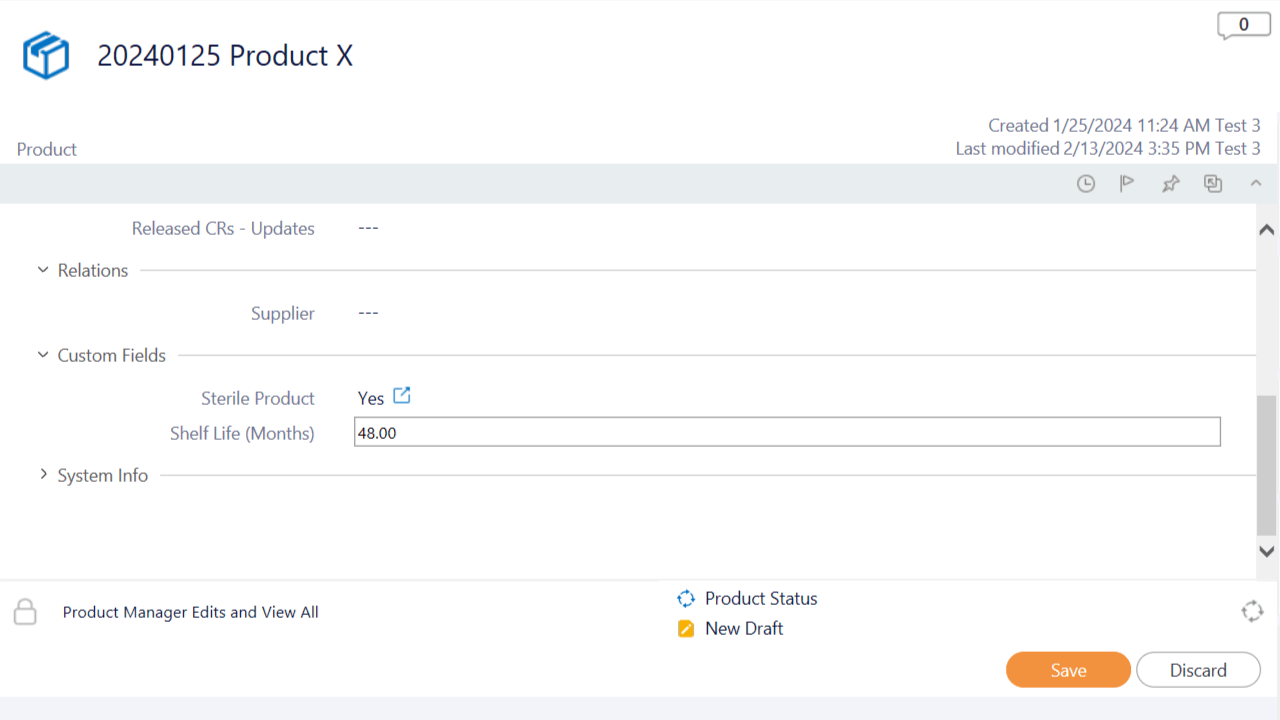
Utilize Custom Product Data Fields
Implement custom fields to capture supplementary details about products. Ensure a thorough and customized documentation approach that goes beyond pre-defined information. For example, use custom fields to capture details related to product sterilization and the shelf life.
Customize additional data fields to precisely meet your product information requirements using lists, numbers, dates, and text fields.
Integrate Into Other Systems
Integrate SimplerQMS with existing systems via open API and plugins, including PLM, ERP, CRM, LMS, and more.
Leverage the functionality of our open API to tailor integrations according to your specific needs, ensuring a flexible and customized approach to system connectivity.
Product and Components Handling in Action
Link all relevant files to a chosen product and enjoy easier compliance.
In this video, we uncover how SimplerQMS relates components and documents to a specific product with a variety of options for documentation.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Complete QMS Software for Life Sciences
Document Management
Maintain control over all quality and regulatory documentation with centralized and secure management.
Change Management
Implement changes in your QMS effectively without compromising on structure or compliance.
Complaints Management
Handle customer complaints efficiently with streamlined tracking and resolution processes.
Audit Management
Reduce the effort and time needed to prepare for and undergo audits with efficient audit management tools.
Nonconformance Management
Record, evaluate, analyze, and manage non-conformances throughout your organization.
CAPA Management
Manage CAPA activities seamlessly from identification to resolution and reporting.
Frequently Asked Questions
What Is Product Management?
Product Management is the process of overseeing and controlling the actions related to the development, documentation, and lifecycle of products within an organization.
Product Management aims to improve risk identification and mitigation strategies, ensure safety to the end user, support compliance with requirements, improve overall system efficiency, and foster a culture of continuous improvement in managing and developing products.
What Are the Main Features of Product Management in QMS Software?
The main features of Product Management in Quality Management System (QMS) software typically include the following.
- Document Control: Helps ensure all product-related documents, such as specifications, manuals, and design documents, are systematically controlled and maintained to guarantee accuracy and compliance.
- Change Control: Allows control of changes to product specifications, designs, or processes, ensuring that modifications are documented, reviewed, and approved to maintain product quality and regulatory compliance.
- User Role and Access Management: Enables management of user roles and access permissions to ensure that only authorized personnel have access to specific product information and functionalities, maintaining data security and compliance.
- Workflow Automation: Streamlines product-related workflows, from design changes to approvals, to enhance efficiency, reduce manual errors, and ensure consistent processes across the product lifecycle.
What Are the Benefits of an eQMS With Product Management Capabilities?
The benefits of QMS software with Product Management capabilities include the following.
- Risk Mitigation and Product Safety: Utilize product management capabilities to proactively identify and address potential risks. By integrating risk management into the product lifecycle, organizations enhance product safety, ensuring a secure experience for end users.
- Centralized Product Information: Have a single source of truth for all product-related information, enhancing accessibility and organizational efficiency. Centralized documentation management ensures consistent, accurate information across the organization.
- Related Data: Relate product data effortlessly within the system by linking products to various elements, including documents, suppliers, risk assessments, CAPAs, and more. Simplify regulatory submissions and facilitate document organization.
- Cross-Departmental Collaboration: Foster collaboration by enabling real-time access to product data across departments. Break down silos and streamline communication to enhance overall teamwork and coordination.
- Scalability: Effortlessly scale your product inventory to meet the demands of a growing portfolio. A cloud-based system ensures that storage capacity evolves with business requirements.
- Improved Compliance: Reduce errors and maintain compliance by eliminating outdated product documents. Ensure consistent and accurate information, protecting product integrity and complying with requirements.
How Much Does QMS Software With Product Management Cost?
SimplerQMS product management module is part of an all-in-one eQMS solution, encompassing all QMS modules, system implementation, user training, ongoing support, validation, cloud hosting, and more.
The number of licenses acquired determines the pricing structure for the SimplerQMS solution. SimplerQMS offers a subscription model where all features and services are included in the subscription price, eliminating the need for additional costs.
For a detailed view of license types, features, and included services, please refer to our pricing page.
How Long Does It Take to Implement an eQMS With Product Management?
The full SimplerQMS solution, with the product management module, can take around five to six weeks to implement.
SimplerQMS uses a flexible implementation process in phases, deploying different QMS modules in each phase, such as product management, document management, change control, training, and more. The module sequence can be adjusted to meet each customer’s specific operational needs.
For instance, manufacturing organizations may prioritize the Product Management module and implement it sooner in the sequence, after the necessary modules for optimal functioning.
The SimplerQMS team closely collaborates with customers throughout each phase to ensure the system is precisely configured. Support for all implementation phases is included at no extra cost.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.