Quality Audit Management Software for Life Sciences
Reduce the time and effort needed to pass audits successfully by streamlining audit-related activities.
TRUSTED BY
























Quality Audit Management Software Within a Complete eQMS
The audit management solution by SimplerQMS offers an integrated set of tools to support all steps in the audit management process.
The software comes with a complementary document template package that you can use to create supplier, regulatory, external, and internal audits. You can create audit plans, schedule audits, assign Issue Handlers, attach evidence concerning audit findings, and escalate them to CAPAs, if necessary.
SimplerQMS audit management software solution is designed to help companies save time, increase efficiency, and provide support for compliant audit management processes. It is part of a complete QMS solution, which includes all core QMS modules for the life science industry such as training management, nonconformance management, CAPA management, supplier management, document control, and more.
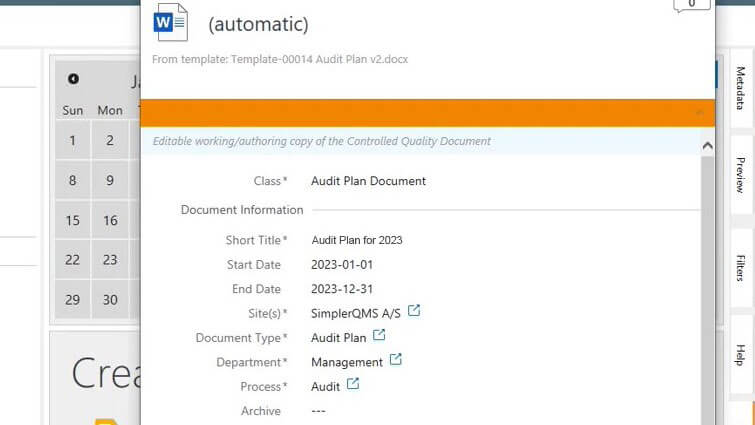
Prepare Audit Plans in Advance
Easily create audit plans using the complementary document template package based on life science requirements. You can also customize them and/or create your own templates to meet specific needs.
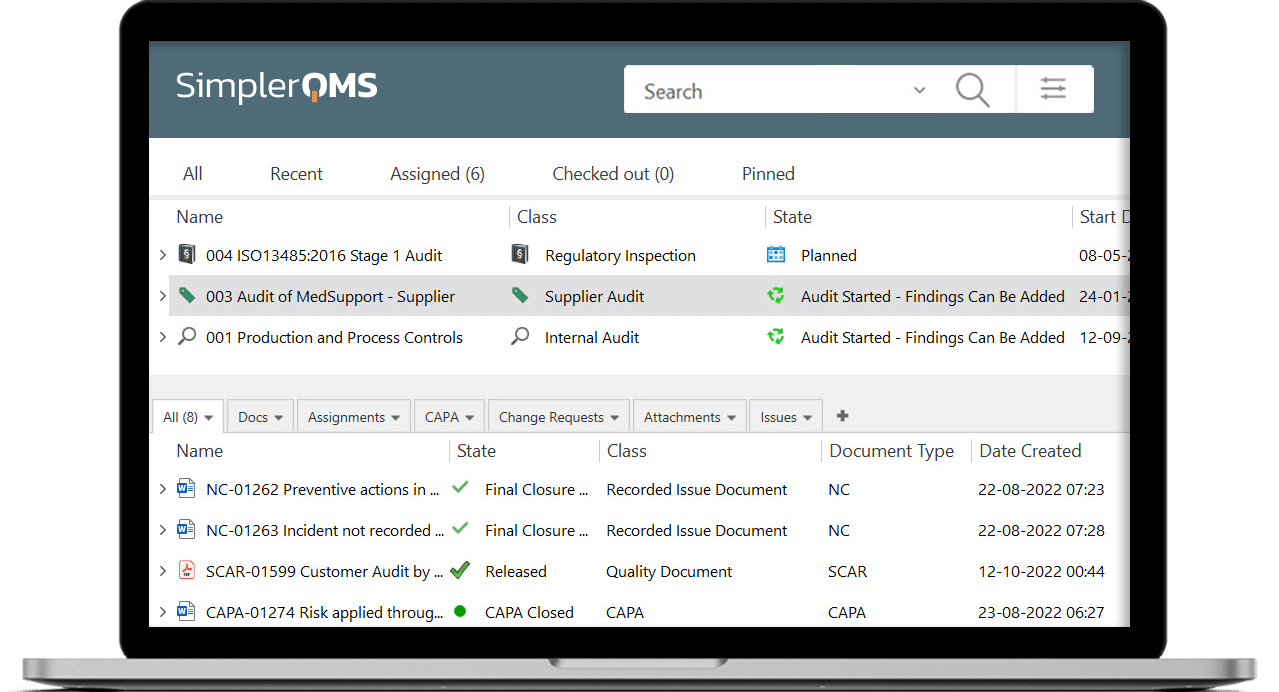
Structure audit plans by filling out the metadata card – set title, site(s), department, process, responsible person(s), author(s), reviewer(s), approver(s), assignments, and so on.
Create and link individual audits (regulatory inspections, supplier audits, internal and external audits) to the audit plan to keep track of all scheduled audit-related activities. Add as many audit findings as necessary and view all information of a specific audit in the metadata card.
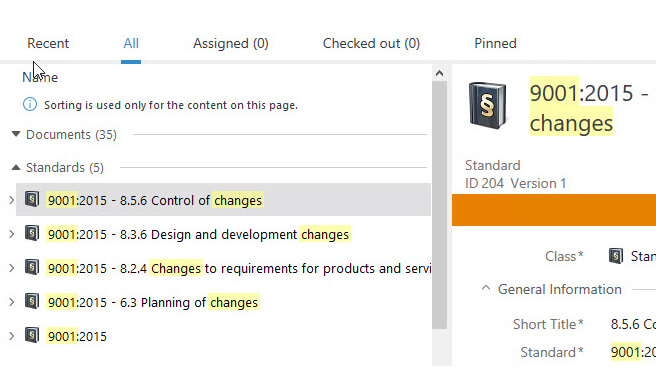
Ensure Regulatory Compliance
Utilize SimplerQMS document templates and pre-configured workflows designed for life science organizations.
SimplerQMS provides document control functionality which ensures that only the most up-to-date version of a document is always used. The system automatically records all changes in documents and creates a complete, time-stamped audit trail.
Store all data in a single cloud-based system to keep all documents in one place. Retrieve specific documents inside the system during an audit situation. Search for exact words that match the titles and content of the document.
Handle Audit Findings With Ease
SimplerQMS audit management solution makes it easier to handle and track the resolution of identified issues. Create audit reports and link all documents to ensure the traceability of information.
Assign workflow participants, such as QA responsible people and Issue Handlers, to approve audit drafts and evaluate CAPA necessity.
Escalate audit finding to CAPA with just a few clicks and get visibility into the progress of each CAPA. The system automatically notifies relevant persons of required activities before the due dates.
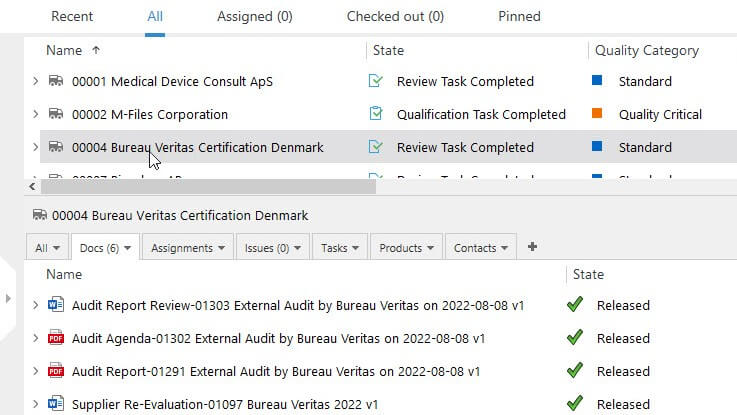
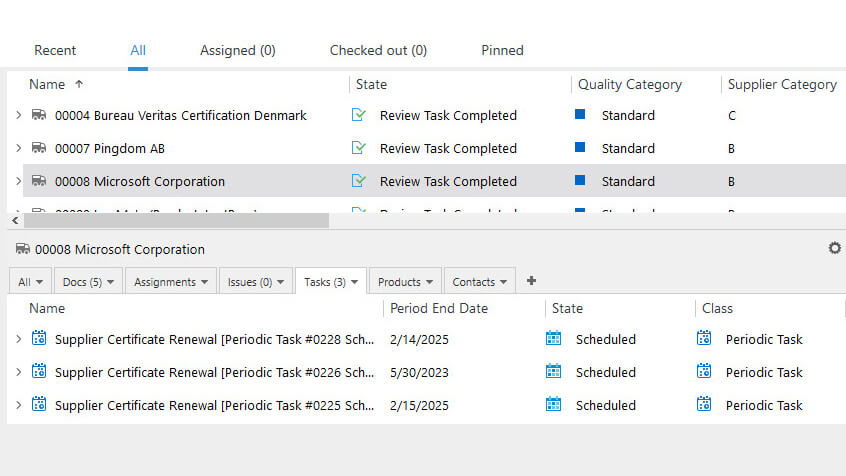
Reduce Supplier Audit-Related Activities
Plan, schedule, and manage supplier quality audits with ease – assign relevant personnel, keep track of all activities, and handle all audit-related records and documentation in one system.
Set reminders to automatically notify relevant people of upcoming scheduled audits or expiration dates of certificates.
Easily add evidence of nonconformities with document attachments in any format, such as images, tables, PDFs, texts, and so on.
Send supplier audit reports and SCAR (Supplier Corrective Action Reports) to your suppliers, if necessary.
Drive Continuous Improvement Across the Organization
Analyze and learn from audit findings to prevent issues from happening again and drive continuous improvement across your organization.
Escalate audit findings to a CAPA, track and manage Corrective Actions and Preventive Actions (CAPA) to closure.
Drive continuous improvement across the organization, from equipment management and training management to supplier management and document control. Audit management software by SimplerQMS interlinks with all QMS modules in the system, including the aforementioned CAPA management, training management, document control, and many more.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Complete eQMS Software for Life Sciences
Supplier Management
Streamline all supplier-related activities for better oversight and control of your supply chain.
Training Management
Train, remind, and assess the training effectiveness of the entire personnel involved in the process.
Nonconformances
Provide fast and accurate resolutions to nonconformances and identify areas for improvement.
Document Control
Ensure all documents are correctly created, approved, stored, and versioned.
CAPA Management
Implement preventive and corrective actions seamlessly to ensure issues do not happen again.
Change Management
Simplify change requests for documents, templates, products, and processes.
Frequently Asked Questions
What is Quality Audit Management Software?
Quality audit software is a software solution that helps organizations to manage the audit process, including scheduling, assigning personnel, monitoring actions, tracking progress, and creating quality audit reports.
It enables organizations to track audit findings and quickly review, evaluate, address any non-conformances, and escalate them to corrective and preventive actions (CAPA) when needed.
SimplerQMS audit management software is part of a complete eQMS solution that includes all core life science QMS modules, such as nonconformance and CAPA management, supplier management, training management, document control, change control, and others.
What Are the Benefits of Audit Management Software?
Audit management software offers numerous advantages for organizations of any size. It enables users to easily schedule audits and assign relevant personnel to the workflow, as well as automate notifications for upcoming tasks and assignments.
The solution enables the gathering of evidence of nonconformities and attachments in any format. Moreover, audit findings can be quickly escalated to CAPAs and coupled with interconnected QMS modules for better QMS oversight, such as training management, document control, supplier management, and many more. These advantages increase the accuracy and efficiency of the audit management processes, improving organizational effectiveness, performance, and compliance.
How Can Audit Management Software Help Meet Regulatory Requirements?
Audit management software supports life science organizations to meet requirements by identifying the gaps between real-world practices, process effectiveness, and requirements.
The system offers features that simplify the audit management process, such as creating audit plans, creating individual audits, keeping track and reporting on findings and actions, and maintaining the audit history.
Robust and straightforward document retrieval capability also enables organizations to easily access the documents needed, for instance, during an audit, reducing the time and effort required to find them.
How Much Does Implementing an Audit Management Software Cost?
SimplerQMS audit software is part of a complete Electronic Quality Management System (eQMS), which includes all life science QMS modules, system implementation, user training, ongoing support, continuous re-validation, cloud hosting, and more.
All SimplerQMS features and services are included in the price, so no other costs are involved. As a result, the total cost will vary according to the number of licenses purchased.
See our pricing page for more information and contact us to get a demo and a quote today.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.