Document Control Software for Life Sciences
Keep your documents audit-ready while managing a high volume of data accurately.
TRUSTED BY
























Robust Document Control Built Into an All-In-One eQMS Solution
The SimplerQMS Document Control Module is only one of the many features built into the solution. We provide an all-in-one, cloud-based quality management software to help life science companies to work more efficiently with documentation, and achieve and maintain compliance with regulatory requirements.
The SimplerQMS solution includes essential QMS modules such as change management, design control, audit, supplier, non-conformance/deviation, CAPA management, and much more.
Let’s see how Document Control Software can benefit you today.
Work Efficiently in Microsoft Office
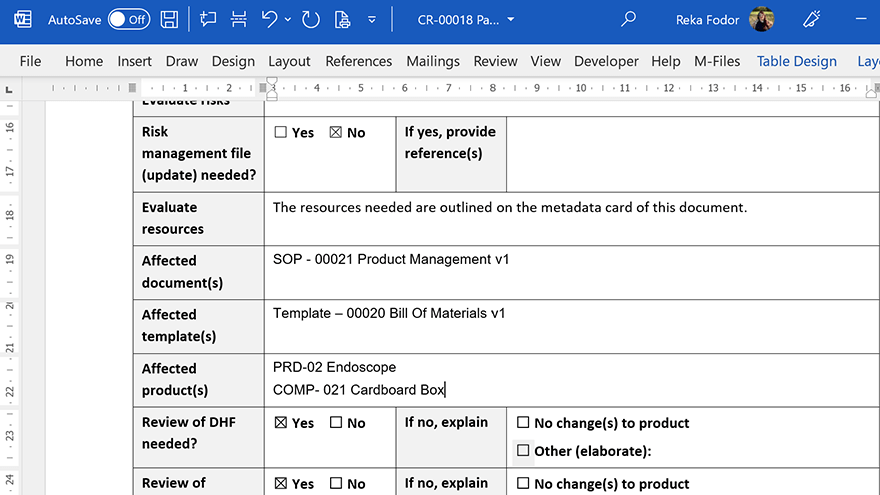
SimplerQMS Document Control software allows you to integrate Microsoft Office applications, so you can keep using the familiar Word, Excel, and PowerPoint applications to work on your documents.
Streamline document creation and organization with document templates.
Work with metadata-driven technology that enables setting relations, names, and versions of files automatically.
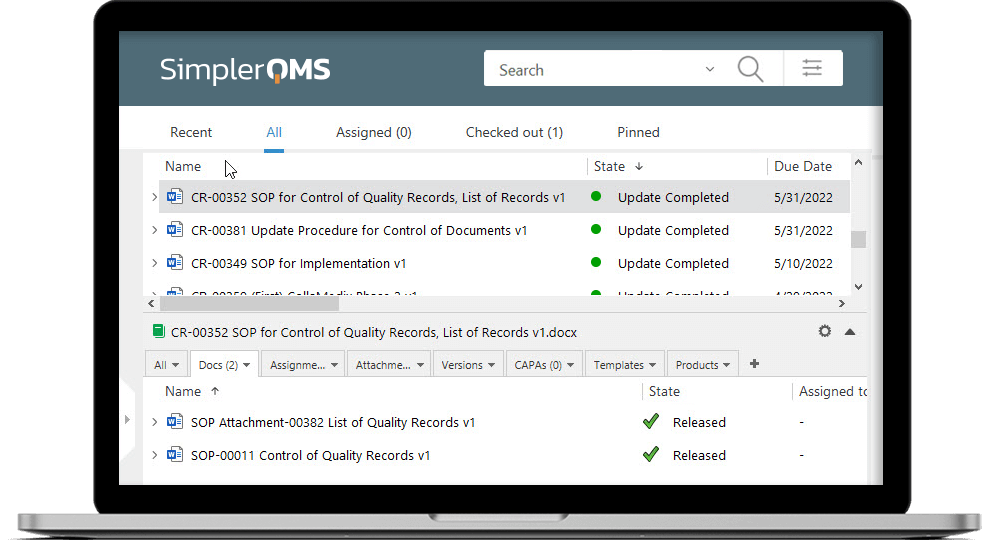
Manage All Documents in a Cloud-Based Solution
Companies using paper-based or hybrid QMS often worry about misplacing or losing files and binders.
SimplerQMS Cloud-Based Document Control Software stores data in the state-of-art Microsoft Azure Cloud Data Centers, which allows your information to be stored securely within an ISO 27001:2022 and FDA 21 CFR Part 11 compliant system.
Enjoy the seamless experience of working on documents directly in the SimplerQMS and have them in order and readily accessible anywhere. Even on your phone, when visiting a supplier.
Out-of-the-Box Regulatory Compliance
SimplerQMS supports compliance with Life Science requirements, such as GxP, ISO 13485:2016, 21 CFR Part 820, ISO 9001:2015, EU MDR and IVDR, 21 CFR Part 11 and EU GMP Annex 11, and others.
We provide a fully validated system according to GAMP5, with continuous re-validation of the software every month. Furthermore, SimplerQMS is fully compliant with ISO 13485:2016, 21 CFR Part 820, 21 CFR Part 11, and the EU Annex 11 regarding validation and electronic signatures.
Be at ease with document control to focus on what is essential: the document’s content. After all, this is the information that matters to achieve compliance.
Utilize Best-Practice Document Templates
Sometimes paper-based or hybrid systems can be prone to errors, such as incomplete forms, documents with incorrect information, and delays in updates.
SimplerQMS software provides built-in templates to automate relations and help you create accurate documentation.
Customize our templates and/or create your own for a more personalized experience.
Protect Documents From Unauthorized Access
SimplerQMS offers a hierarchy of control over documents, so only specific employees can access certain files.
Set access levels to specific sites, projects, document types, such as SOPs, Meeting Minutes, and Work Instruction, and more. Determine the author, reviewer, and approver access to a document. Even select entire departments to read and learn the file once it is released.
Assign a specific person as an approver when creating a new document, such as a CAPA or SOP, to ensure that only that person can release the document, not someone else.
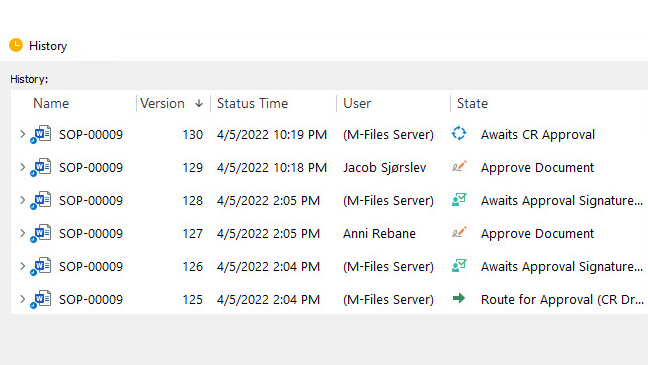
Simplify Review and Approval Processes
Using paper-based and hybrid systems can cause a delay in releasing documents. The order of priority can be confusing, and documents can even be forgotten entirely.
SimplerQMS software help streamline the process of reviewing and approving documents.
Set workflows and use email notifications to keep all team members aware of tasks and assignments regarding specific documents.
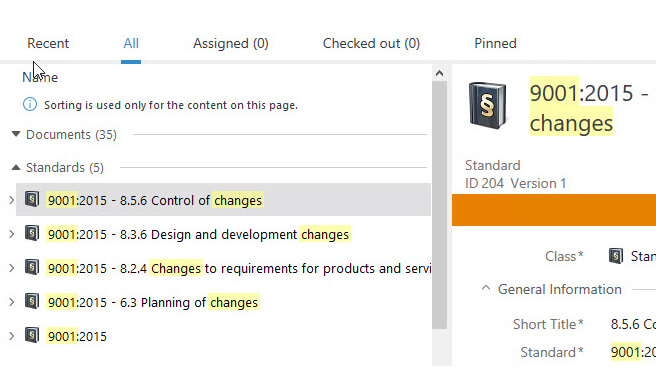
Relate Documents in One Step
SimplerQMS solution enables documents to be easily linked to one another or specific departments and standard chapters.
Set relations using the Document Control Software metadata to help streamline audits. A simple search in the system can quickly retrieve all documents related to a requirement.
A new procedure document can be related to the existing Quality Manual document and appointed to the specific ISO chapter in a single step during document creation.
See Our Document Control Capabilities in Action
SimplerQMS seamlessly integrates with familiar Microsoft Office applications – Word, Excel, Outlook, and PowerPoint. Work on your documents in Microsoft Office and store them with one click in SimplerQMS Cloud.
What Customers Achieve With SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass audit more easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain high level of traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Beyond Just Document Control Solution
Change Management
Monitor all changes to documents and ensure compliance within your organization’s QMS.
Audit Management
Retrieve documents easily and be audit-ready all the time using our fully validated QMS software.
Supplier Management
Handle supplier documentation following standards and simplify supplier-related activities.
Training Management
Provide training activities, set reminders, create tests, and issue certificates to facilitate learning.
Complaint Management
Provide fast and accurate resolutions to complaints and identify areas for improvement.
CAPA Management
Identify and reduce risks by implementing preventive and corrective actions seamlessly.
Frequently Asked Questions
What Is Document Control Software?
Document control software is a form of software that helps to author, organize, store, and track the documents used within an organization. It simplifies document processes such as creating, editing, sharing, and approving documents.
In the highly regulated Life Science industry, an eQMS solution with powerful document control capabilities is essential to help you achieve compliance with regulations and standards.
SimplerQMS is an all-in-one QMS software solution. Besides robust document control features, we offer core QMS modules such as deviation/non-conformance and CAPA management, design control, change, supplier, audit Management, and many more.
What Are the Benefits of Document Control Software?
Implementing Document Control Software gives you the benefits of:
- Keep working on the familiar Microsoft Word, Excel, and PowerPoint
- Manage all documents from one centralized location
- Access your documents anywhere
- Utilize a fully validated system
- Use built-in templates to create documents
- Protect documents from unauthorized access
- Simplify the review and approval process
- Easily interlink documents to any other data
Who Is Going to Have Access to the Documents?
Only the employees you designate will have access to documents. With the SimplerQMS solution, you can define access levels and protect specific documents.
Are There Document Templates and Forms Available?
SimplerQMS software has built-in templates for standard procedures, complaints, change requests, CAPA, risk management plans, and more.
Also, you can create new templates and customize your documents however you like.
Can I Use Electronic Signatures to Sign Documents?
SimplerQMS allows you to sign any document at any time, from anywhere with 21 CFR Part 11 and EU GMP Annex 11 compliant electronic signatures.
Will My Documents Be in Compliance With Regulations?
SimplerQMS supports you in achieving compliance with essential Life Science regulations and standards.
Nevertheless, users are responsible for ensuring that the content of documents is compliant.
Also, our software is entirely validated according to GAMP5. And complies with ISO 13485:2016, 21 CFR Part 820, 21 CFR Part 11, and EU GMP Annex 11 regarding electronic signatures and validation.
How Can Document Control Solution Make the Audits Easier?
Document Control Software allows you to relate documents, making it easy to search and retrieve important forms during an audit process.
Also, all document history is available with time-stamped audit trails.
How Much Does Document Control Software Cost?
SimplerQMS Document Control Software is part of an all-inclusive solution for eQMS.
Our system includes several QMS modules, implementation, user training, and ongoing support in the same license. This way, the total cost will vary depending on the number of licenses you acquire.
If you are interested, please visit our pricing page.
How Much Time Does It Take to Implement Document Control Solution?
The time to implement Document Control Software can vary depending on the volume of documents you have to migrate or create in the software and the resources available.
Usually, it takes 5 to 6 weeks to implement the SimplerQMS solution.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.