Regulatory Compliance Management Software
Meet continually evolving regulatory compliance requirements and deliver compliant products to the market faster.
TRUSTED BY
























What’s in SimplerQMS Regulatory Compliance Management Software?
SimplerQMS provides compliance management software integrated into a cloud-based quality management solution suite. The software is designed to help life science companies to work more efficiently and meet the most complex regulatory compliance requirements, including those applicable to medical device, pharmaceutical, and other organizations. The following are some of the core benefits and key features.
Utilize Powerful Document Control Features
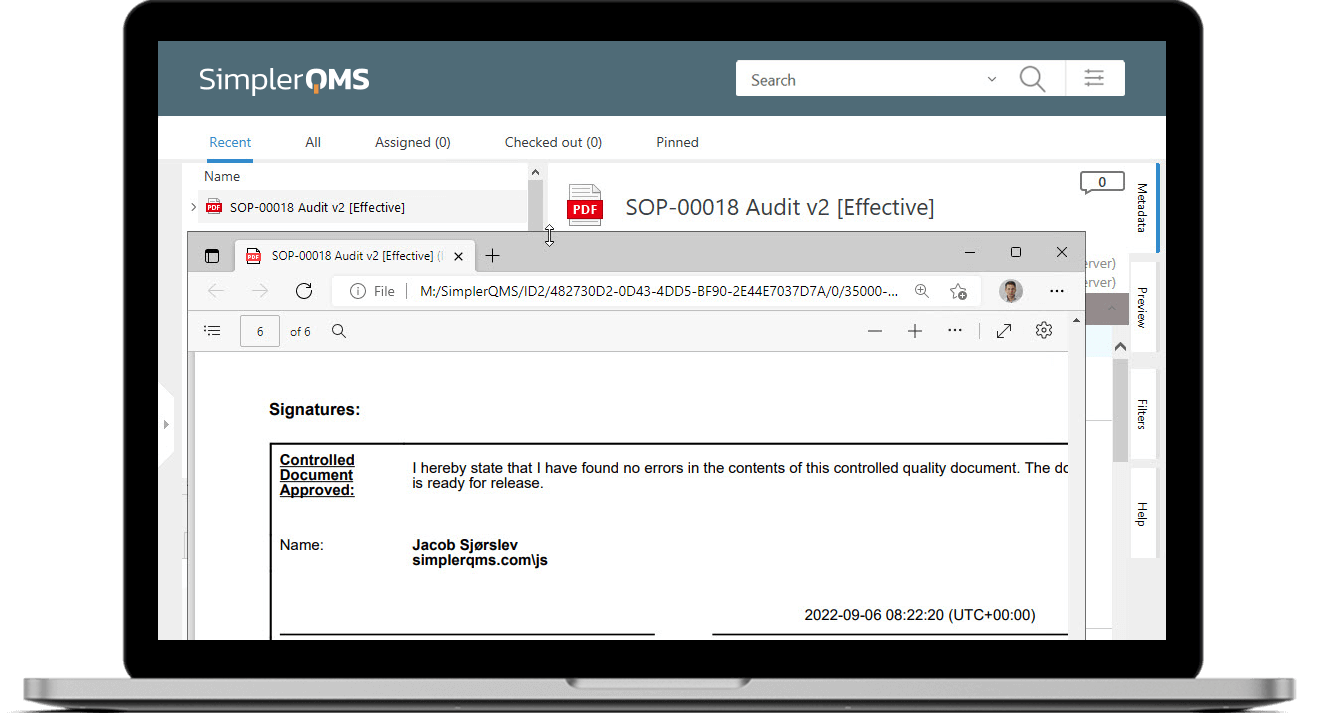
Document control is a critical part of regulatory compliance. Manual paper-based or hybrid document control systems are often insufficient in today’s fast-paced environment and can lead to regulatory compliance risks.
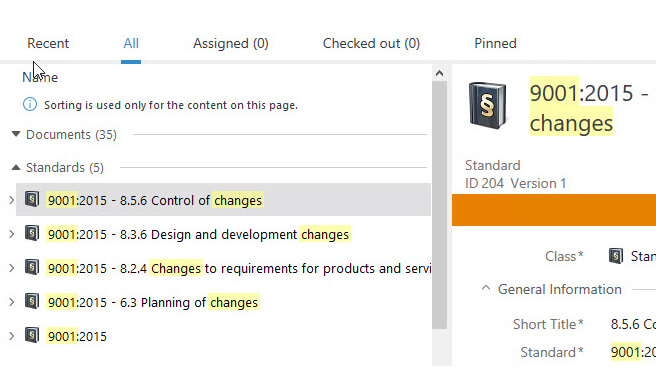
SimplerQMS provides a centralized, cloud-based repository for all your quality and regulatory documents. Streamline and automate your documentation processes with automated workflows for document creation, routing, review, approval, escalation, notifications, reminders, and more.
Easily search and retrieve documents using keywords, document type, or other criteria. Ensure that you always have the most up-to-date documents at your fingertips.
Out-Of-The-Box Regulatory Compliance
SimplerQMS helps you meet regulatory standards and regulations applicable to life science companies, including 21 CFR Part 11, ISO 13485, cGMP, GxP, and others.
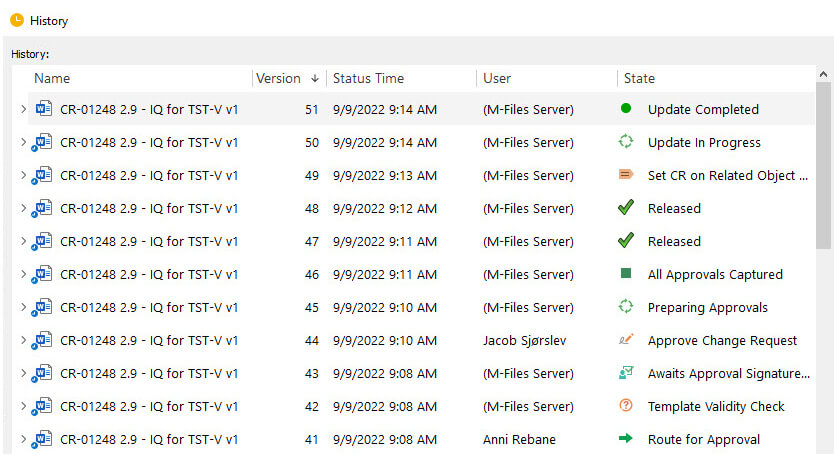
The software provides full, time-stamped audit trails, version control, and electronic signatures compliant with the FDA’s 21 CFR Part 11. Additionally, SimplerQMS comes with preconfigured workflows for common quality processes, such as change control, deviation and non-conformance management, CAPA management, risk management, and more.
This helps you to get up and running quickly and start meeting regulatory compliance requirements from day one.
Save Time and Money on Software Validation
Traditional validation of compliance software systems – the process of manual testing and documentation – is both expensive and time-consuming. However, it does not need to be that way – you can save time and money by choosing a software solution that is already pre-validated.
SimplerQMS is pre-validated and complies with the FDA 21 CFR Part 11, ISO 13485, FDA 21 CFR Part 820, and GxP guidelines. Furthermore, we ensure continuous re-validation and provide extensive validation evidence which you can use during audits and inspections.
This means that you don’t need to spend any of your valuable resources on manual testing or documentation processes because we have it all done for you!
Enable Closed-Loop Integration With Your Entire QMS
The compliance software solution should not be siloed from the rest of your quality management system (QMS). To streamline quality and compliance, it is essential to have all of your regulatory and quality data – from different processes and departments – in one place.
This is why SimplerQMS provides a closed-loop integration with your entire QMS.
Streamline and connect all processes that are critical to regulatory compliance, including document control, training management, change control, audit management, customer complaints, NC, CAPA, supplier management, and more.
Solution That Streamlines Quality & Compliance
Document Control
Automate and standardize the process of creating, storing, and managing all your documents in one place.
Change Control
Be confident that all changes made to your QMS are documented and approved, ensuring compliance with regulatory requirements.
Employee Training
Automate and streamline the entire employee training process to be faster, more cost-effective, and more compliant.
CAPA Management
Integrate your CAPA management process with the entire product life cycle for a comprehensive view of your organization’s QMS.
Supplier Management
Streamline supplier documentation processes, track supplier performance, and ensure supplier quality and compliance.
Audit Management
Automate many tasks related to audits, from creating and managing audit schedules to compiling data, generating reports, and more.
Frequently Asked Questions
What is Regulatory Compliance Management Software?
Regulatory compliance management software is a type of software that helps organizations operating in highly regulated industries including medical devices, pharmaceuticals, biotechnology, and others to ensure compliance with regulatory standards applicable to their organization.
The software helps streamline processes and integrate the controls and requirements set forth by regulatory bodies into the different business operations.
Typically, regulatory compliance software is divided into several categories based on industry-specific regulations. In the context of life sciences, such software helps companies meet requirements set forth by ISO 13485, FDA 21 CFR Part 820, FDA 21 CFR Part 11, cGMP, GxP, ICH Q10, EU Annex 11, and others, depending on the regulatory jurisdictions they operate in.
What Are the Benefits of Regulatory Compliance Management Software?
Reduced risk of non-compliance: The software helps you stay on top of the various regulatory requirements that are relevant to your business. It helps you ensure that applicable regulatory requirements are integrated into your business processes. This, in turn, reduces the risk of non-compliance, and associated penalties, and protects your business reputation.
Improved efficiency and quality: The software solution centralizes regulatory data and documentation. This makes it easier to access the information you need when you need it. The software also automates workflows, eliminating the need for manual processes. This saves time and improves efficiency and quality across different departments in your organization.
Increase customer satisfaction: By ensuring compliance with regulatory requirements, you can increase customer satisfaction and confidence in your products or services. As a result, you will be able to improve customer retention and attract new customers.
What Are Typical Compliance Management Software Features?
Automated document workflows: The software should at the minimum automate the creation, review, approval, and distribution of documents.
Electronic signatures: The software should allow for electronic signatures compliant with the FDA 21 CFR Part 11.
Full audit trails: The software should maintain full, time-stamped audit trails of all data and documentation.
Version control and history: The software should maintain the version control and history of all documents.
Integration to QMS processes: The most comprehensive solutions allow for integration with other quality management system (QMS) processes, such as document control, change control, audit, supplier, customer complaints, training management, NCs, CAPAs, and more.
How Much Does Compliance Management Tools Cost?
SimplerQMS regulatory compliance management software is part of an all-in-one quality management solution suite. The SimplerQMS subscription, allows you to streamline regulatory compliance activities, it includes all QMS system modules, implementation, training, ongoing support, validation, hosting, and more. This means that you don’t have to worry about any extra costs associated with using the software.
To learn more about how SimplerQMS can help your organization and approximate pricing, request a tailored demo. One of our quality and compliance experts will be in touch to discuss your specific needs and provide you with a personalized quote.
Is Your Compliance Management Software a Good Fit for My Organization?
The SimplerQMS quality and compliance management platform can scale to meet the needs of businesses of all sizes.
Whether you have a team of ten or a large enterprise, our platform scales with your specific needs. Request a demo today to see how our software can benefit your organization.
What Customers Achieve With SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass audit more easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain high level of traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.