Quality Document Management System Software
Create, co-author, approve, change, and train on quality documents within a single platform.
TRUSTED BY
























What’s in SimplerQMS Quality Document Management Software?
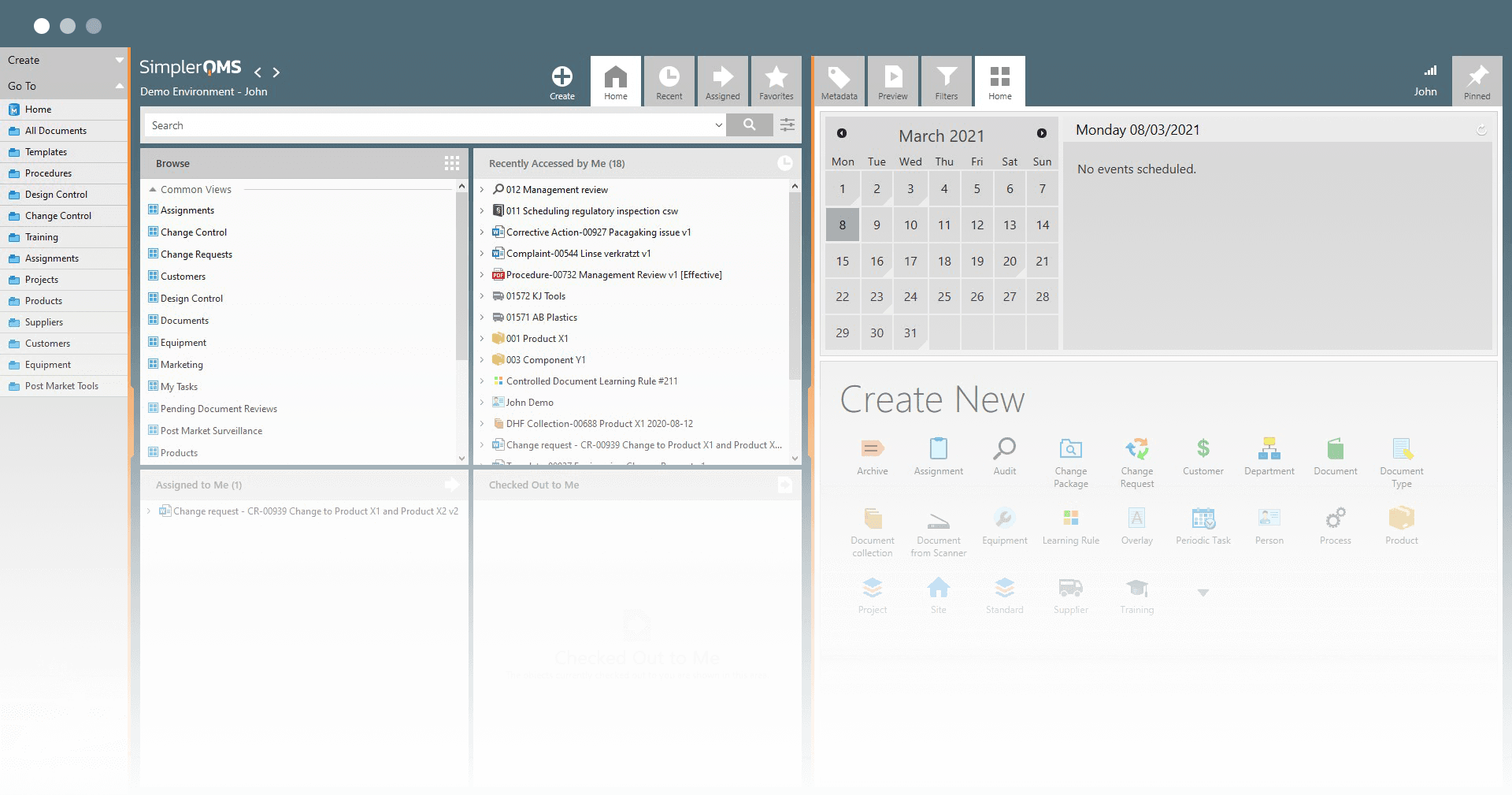
SimplerQMS provides a cloud-based quality document management software that helps life science organizations streamline quality management processes, work more efficiently, and ensure compliance with regulatory requirements. Here are some of the core quality document management features and benefits you get using SimplerQMS.
Streamline Quality Documentation Processes
Approving quality documents is critical to the success of any business, but ensuring that these approvals happen in a timely and efficient manner can be difficult.
SimplerQMS allows you to create documents using best-practice forms and templates. Easily send documents through automated workflows for review and approval processes based on user roles and responsibilities.
Track the progress of quality document approvals, identify any bottlenecks in the process, and escalate overdue activities with ease.
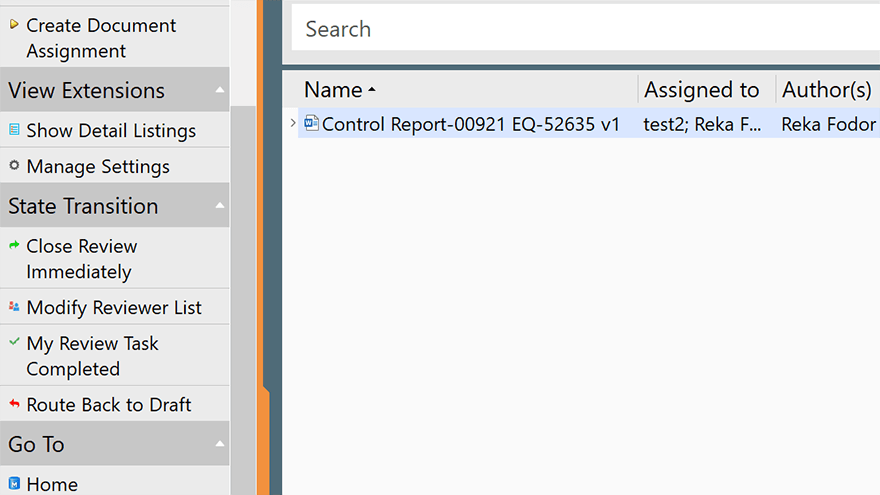
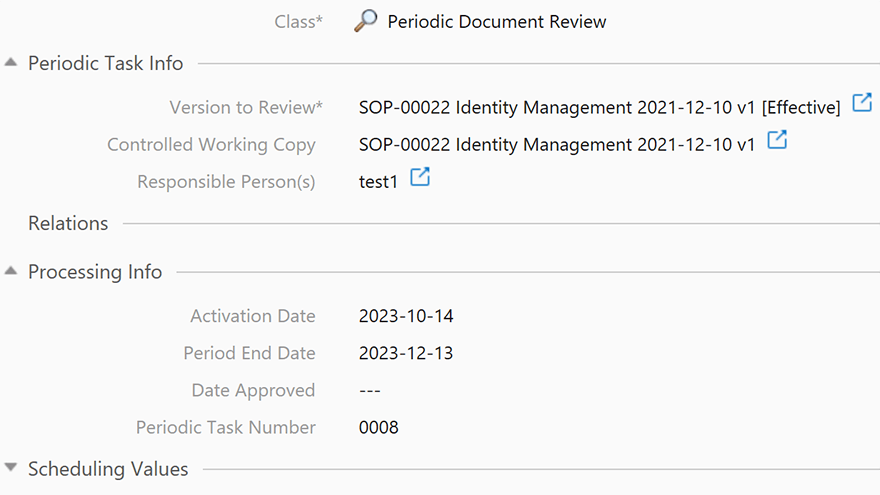
Schedule Periodic Review, Revision, and Reapproval Tasks
Enjoy peace of mind knowing that your quality documents are kept up-to-date.
SimplerQMS allows you to schedule periodic review, revision, and reapproval tasks. Set up alerts and notifications to remind users when a document is due for review or update.
This helps ensure that all quality documents are accurate and compliant with the latest regulations and standards.
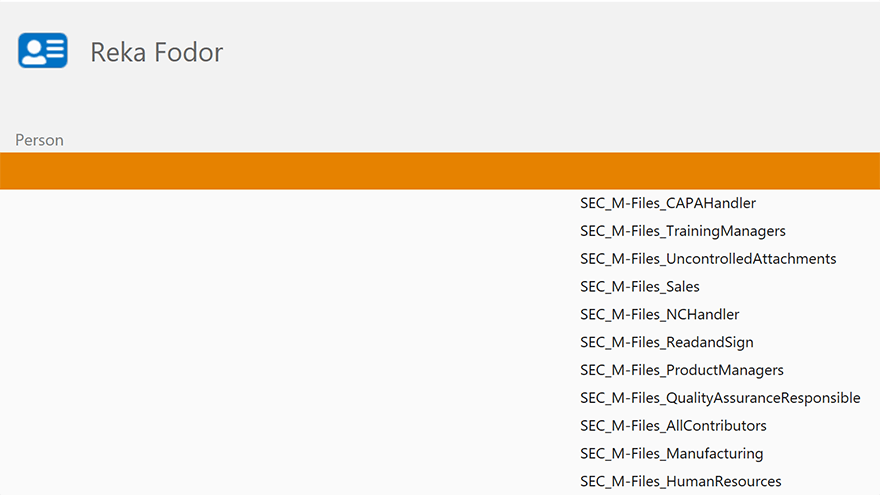
Enforce Quality Document Access Controls
Who should have access to which quality documents?
It’s a question that all organizations must answer to ensure the security and privacy of sensitive information.
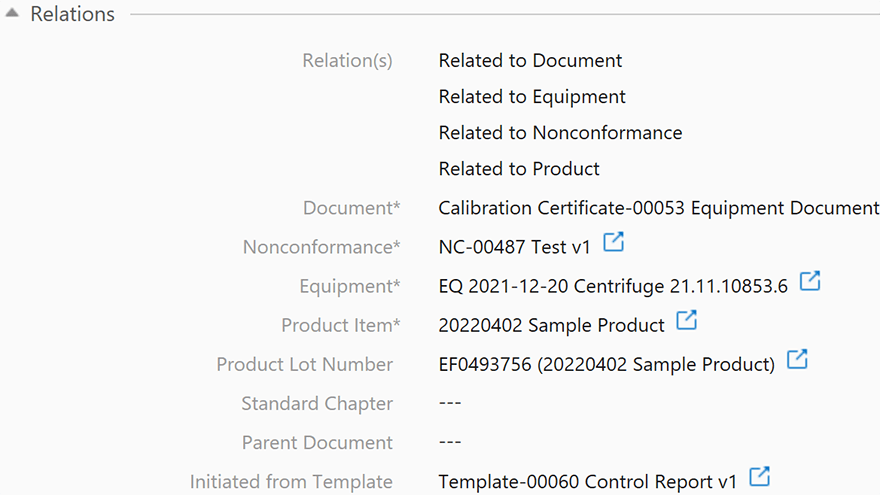
With SimplerQMS, you can enforce document access controls based on the group settings of your users and limit access permissions at the site, project, and document type levels. This ensures that only users with the appropriate permissions can view, edit, create, review, approve, or archive documents.
Connect Quality Processes Across the Organization
Quality management can be a complex and time-consuming process, especially when different departments are using different systems to track quality processes.
For example, process changes often require document updates and new training for employees. SimplerQMS helps you connect documentation and training processes, so you can be sure your employees are always up to date on the latest procedures.
From quality audits, and suppliers to CAPAs, customer complaints, and other processes, SimplerQMS helps you connect them all across the organization for greater efficiency and visibility.
Ensure Regulatory Compliance
Organizations operating within life sciences must meet a variety of regulatory requirements, from FDA 21 CFR Part 11 to ISO 13485, and others.
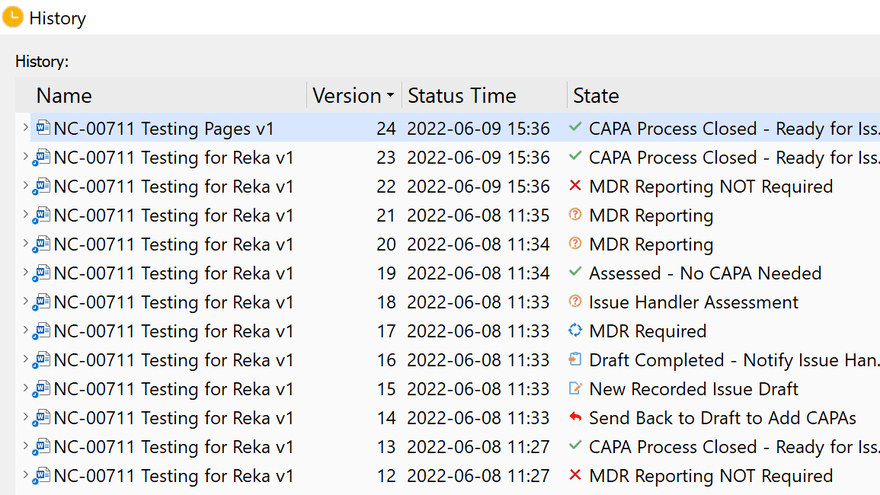
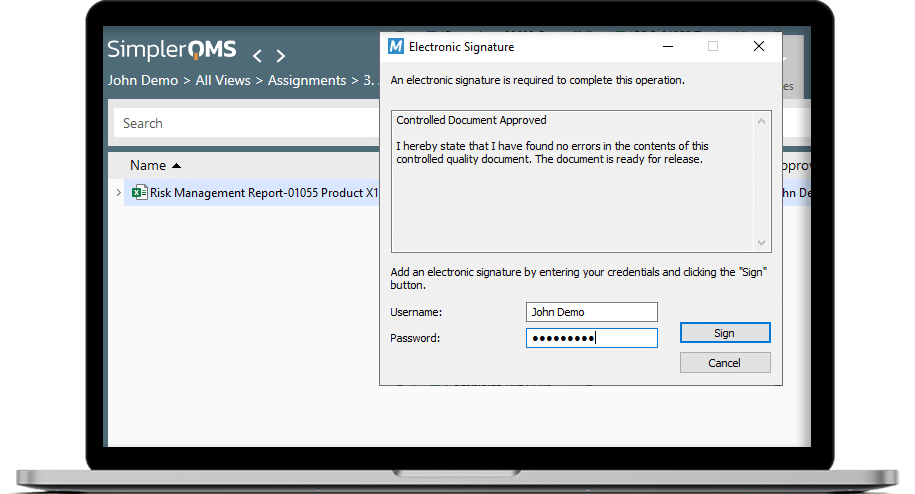
Out-of-the-box, SimplerQMS helps you ensure compliance with the most stringent standards and regulations in the life science industries such as medical device, pharmaceutical, and other industries. With its time-stamped audit trails, versioning and electronic signatures SimplerQMS ensures compliance with 21 CFR Part 11.
Plus, we provide a fully validated software solution and ensure continuous re-validation in compliance with the requirements of GxP, FDA 21 CFR Part 11, ISO 13485:2016.
Work Efficiently in Microsoft Office
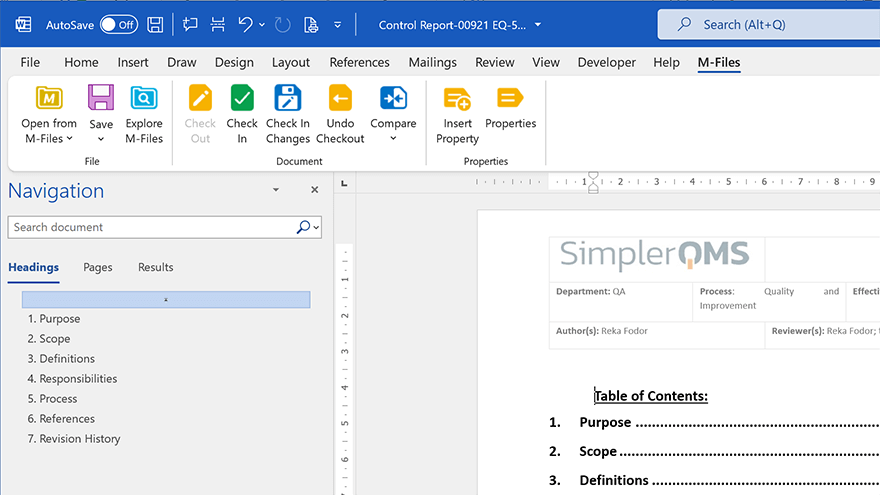
SimplerQMS integrates with Microsoft Office 365 and SharePoint Online, so you can work on quality documents directly in the familiar Microsoft Word, Excel, and PowerPoint applications.
No need to learn a new document editing software application – just continue working on the tools you already know and love.
After the work is done, save documents directly to the Cloud with one click, no need to manually export and upload files.
eQMS That Goes Far Beyond Quality Document Management
Training Management
Automate employee training assignments, reminders, certificate generation, and more, so you can save time and ensure compliance.
Complaint Management
Streamline your customer complaint management processes, improve customer satisfaction, and ensure regulatory compliance.
Non-Conformances
Track and analyze the trending of non-conformances, identify root causes, and take corrective action with ease.
CAPA Management
Streamline the process of identifying, uncovering, resolving, and reporting preventative actions and corrective actions (CAPAs) with ease.
Change Management
Create, edit, review, and approve change requests, keep track of who made what changes, and see the overall impact of the change.
Audit Management
Manage audit-related processes more effectively and efficiently, while reducing the risks associated with manual processes.
Frequently Asked Questions
What is Quality Document Management System?
A quality document management system enables organizations to manage their quality documentation in compliance with regulatory and Quality Management System (QMS) requirements for documentation, including ISO 9001, ISO 13485, FDA 21 CFR Part 11, FDA 21 CFR Part 820, and others.
As the name suggests, quality document management system software helps you manage quality documentation processes throughout their lifecycle while eliminating manual paper processes. With automated workflows, electronic signatures, and centralized document storage, quality document control software system, like SimplerQMS, can help you improve quality and compliance while reducing costs, human error, and improving efficiency.
What Makes Our Quality Document Management Software System Popular?
Popular features of SimplerQMS quality document control software include:
- Centralized cloud-based document repository: Easily find documents with just a few clicks of your mouse. Access them anytime, from anywhere in the world.
- Microsoft Office integration: Native integration with Microsoft Office allows you to work on your documents in Microsoft Word, Excel, and PowerPoint without leaving the system.
- Interlink quality documents with other processes: Easily connect the dots between your subsystems such as suppliers, products, components, non-conformances, CAPAs, quality audits, and others.
- Out-of-the-box regulatory compliance: Time-stamped audit trails, document versioning, FDA 21 CFR Part 11, and GxP compliant electronic signatures. Plus, we provide a pre-validated quality document control solution, which complies with the requirements of the Gx, GAMP 5, FDA 21 CFR Part 11, ISO13485:2016, and ensures continuous re-validation.
- Automated workflows: Automate data collection, document routing, notifications, reminders, follow-ups, escalation of assignments, and more.
Are SimplerQMS Quality Document Control Tools 21 CFR Part 11 Compliant?
Yes! The quality document management tools by SimplerQMS are designed specifically for life science organizations and ensure compliance with the Electronic Signature and Digital Record practices set forth by the US FDA’s 21 CFR Part 11.
How Much Does Quality Document Management System Software Cost?
SimplerQMS quality document management solution is part of an all-inclusive subscription, which includes all core QMS modules, implementation, training, ongoing support, validation, hosting, and more.
This means that everything is included in the price you pay, so you can rest assured that there are no hidden costs associated with subscribing to SimplerQMS.
The cost of the solution depends on how many licenses you need and what type they are. That’s why we recommend booking a demo and talking to our experts for an accurate price quote that will work best with your organization’s needs!
How Much Time Does It Take to Implement a Quality Document Management Solution?
Depending on whether you already have a quality management system (QMS), and the number of documents, implementation time may be shorter or longer.
We take pride in helping our clients launch and start using SimplerQMS quickly so they can focus on their business instead and unlock the value of the SimplerQMS solution faster! The average implementation time is 6 weeks – though it could happen much faster depending upon your situation.
What Customers Achieve With SimplerQMS
“The chance of a human error in the process of filling out documents has been reduced. There are several different human errors that have been eliminated and we feel like the system helps us to catch possible human errors more easily.”

Christian Schärfe Thomsen
Project Manager, Cortex
Ready to learn more?
To learn how your organization can make the most of SimplerQMS, request a personalized demo presentation.