Pharmaceutical Document Management Software
Streamline document management processes, accelerate time to market, and ensure compliance with regulatory requirements.
TRUSTED BY
























What’s in SimplerQMS Pharmaceutical Document Management System?
SimplerQMS provides a cloud-based pharmaceutical document management software that enables efficient and secure management of documents. The software integrates with a quality management software suite to provide a comprehensive solution for pharmaceutical manufacturers. Here are some of the key features and advantages you can expect to see when using SimplerQMS.
Automate Document Management Activities
Paper-based or hybrid document management systems can be time-consuming and error-prone, leading to delays in getting products to the market.
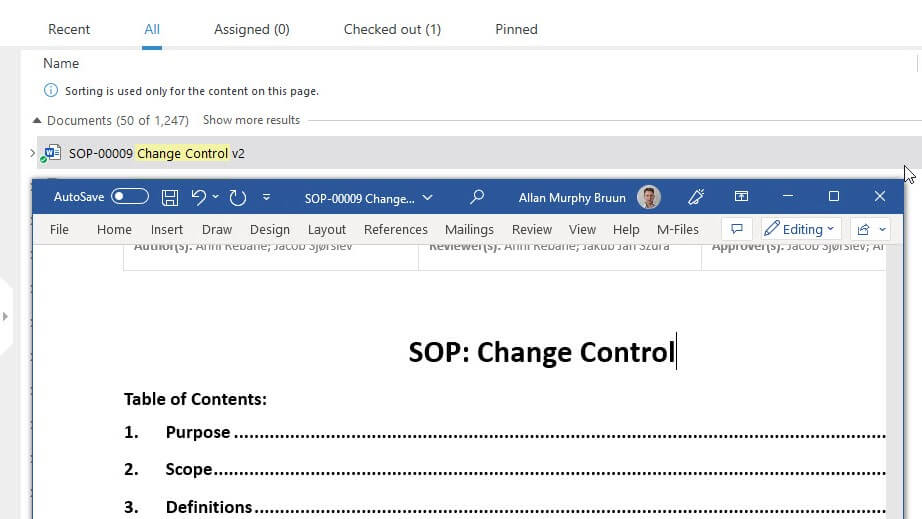
SimplerQMS automates key document management processes, such as document creation, routing, review, approvals, version control, distribution, task reminders, escalation, and notifications.
Create documents using forms and templates in Microsoft Word, Excel, and PowerPoint, and save them in the system with one click. Easily track document status and progress with real-time dashboards and reports.
Promote Efficiency With Electronic Approvals
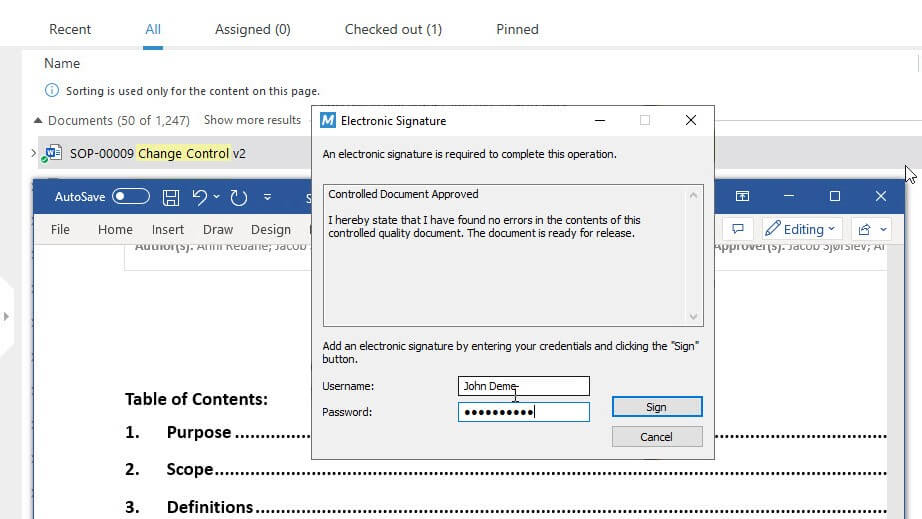
Signing documents electronically saves time and hassle by eliminating the need to print, sign, and scan documents.
SimplerQMS provides FDA 21 CFR Part 11 compliant electronic signatures and full audit trails. Approvers can review and sign documents electronically from any device, anywhere, at any time.
No more wet signatures or paper documents!
Simplify Compliance With Regulatory Requirements
Like most life science industries, the pharmaceutical industry is highly regulated, and manufacturers must comply with FDA guidelines, good laboratory practices (GLP), current good manufacturing processes (cGMP), ICH Q10, EU Annex 11, and others. SimplerQMS is designed to help pharmaceutical manufacturers meet these compliance requirements.
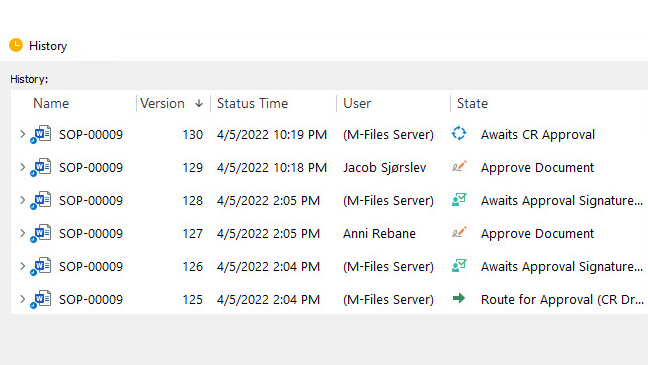
With automatic version control and revision control, you can always be sure that only the most current documents are accessible. FDA 21 Part 11 signatures, time-stamped audit trails for all changes to any document, and a centralized, cloud-based repository will help assure compliance as well as provide ready access during audits and inspections.
Efficiently Control Prints Across the Organization
Controlled Printing in SimplerQMS allows users to generate controlled prints of documents, and track, manage, and recall documents that have been printed.
Controlled printouts contain a unique ID, date, creation time, the responsible person, and the printout type in the footer for easy identification of different document copies. Uncontrolled prints have an additional “COPY” overlay at the top of the document.
Recalls are automatically sent out or you can issue a recall of controlled printouts and mark them as destroyed in the system to ensure outdated documents are not used.
Connect Document Management to Other Processes
Disconnected or siloed quality management processes can lead to inefficiencies, compliance risks, and quality issues.
SimplerQMS pharmaceutical document management solution seamlessly interconnects with key quality processes, such as training, suppliers, change control, deviations, CAPA, customer complaints, audits, and more. The software can also be integrated with popular ERP, CRM, PLM, and other systems through an API.
By integrating document management with other quality processes, you can get a complete picture of your pharmaceutical quality system, identify process improvements, and reduce risk.
All-in-One eQMS for Your Entire Pharma Organization
Training Management
Keep track of who needs to be trained on what, and when, and ensure your employees are always up-to-date on the latest procedures.
Deviation Management
Manage all aspects of deviations, from initial report through to resolution, in one centralized location.
CAPA Management
Identify, uncover, resolve, and report all the preventative actions and corrective actions (CAPAs) within your pharmaceutical organization.
Audit Management
Streamline the entire audit process, from planning, scheduling, and preparation to execution and reporting, and improve your chances of passing audits with flying colors.
Complaint Management
Collect and track customer complaints, manage your team’s response to complaints, and turn negative feedback into positive results.
Supplier Management
Select your preferred suppliers, track and monitor their performance against key metrics, and ensure that they continue to meet your expectations.
Frequently Asked Questions
What is Document Control in Pharmaceutical Industry?
Document control in the pharmaceutical industry is the process of handling and managing documents so that only the most current versions are accessible, changes are tracked, and compliance with regulatory requirements is assured. Document control is a critical part of quality management and compliance.
Recommended Reading: A Guide to Effective Pharmaceutical Document Management
What is Pharmaceutical Document Management Software?
Pharmaceutical document management software is developed to help pharmaceutical organizations facilitate document management processes, save time and meet document control requirements under FDA guidelines, GLP, cGMP, ICH Q10, EU Annex 11, and others.
The software typically includes features such as automated workflows for document creation, review, and approval, as well as task reminders, notifications, and more. Other features include document version control, FDA 21 Part 11 signatures, time-stamped audit trails, and a centralized, cloud-based file repository.
What Makes SimplerQMS Pharmaceutical EDMS Popular?
Popular SimplerQMS document management software features include the following features:
- Pre-validated software solution: The software is pre-validated and comes with the necessary documentation that meets validated computer system compliance requirements.
- Broad QMS process support: The software supports a broad range of pharmaceutical quality management processes, such as training, supplier management, change control, customer complaints, deviations, CAPAs, and audits.
- Microsoft Office integration: The software integrates with Microsoft Office applications, such as Word, Excel, PowerPoint, and others.
- Electronic signatures: The software supports FDA 21 CFR Part 11 electronic signatures.
- Time-stamped audit trails: The software records all changes made to documents, who made the changes, and when the changes were made.
- Cloud-based file repository: The software stores all files in a centralized, cloud-based repository.
Is SimplerQMS EDMS Software FDA 21 CFR Part 11 Compliant?
SimplerQMS is a 21 CFR Part 11-compliant software system that helps Life Science companies comply with the Electronic Signature and Digital Record practices set forth by the FDA’s 21 CFR Part 11.
How Much Do Pharmaceutical Document Management Tools Cost?
SimplerQMS document management software solution for pharmaceutical companies is part of an all-inclusive quality management solution suite, which includes all QMS system modules, system implementation, user training, ongoing support, ongoing software validation, hosting, and more.
This means that everything is included in the price you pay and there are no other costs associated with subscribing to SimplerQMS. The price depends on the number of employees that need to have access to the system.
To find out how much the SimplerQMS solution would cost for your company, request a personalized demo. We will show you how SimplerQMS can help streamline your pharmaceutical documentation and quality management processes and provide you with a cost estimate.
How Much Time Does It Take to Implement an EDMS Solution?
Depending on your existing quality management system and the number of documents, implementation time may vary.
However, we take pride in helping our clients launch and start using SimplerQMS quickly. On average, implementation takes around 6-weeks.
What Customers Achieve With SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass audit more easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain high level of traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.