Standard Operating Procedure (SOP) is a document that provides directions on how tasks and processes should be carried out within a company.
Standard Operating Procedures (SOPs) are an integral part of the routine operations in the pharmaceutical industry, and every department has its SOPs.
SOP management refers to the systematic process of creating, implementing, and maintaining these procedures. Proper management of SOPs helps ensure all procedures are adequately documented, regularly reviewed, and effectively communicated to the relevant personnel.
Having well-defined and well-maintained SOPs helps pharmaceutical companies consistently carry out routine operations, improve process efficiency, have uniform performance, as well as onboard new employees effectively.
This article will discuss the definition of an SOP, its importance, types, guidelines, format, management process steps, best practices, and the role of QMS software for SOP management in the pharmaceutical industry.
SimplerQMS provides eQMS software with robust document management capabilities designed specifically for Life Science companies. Schedule a demo and talk to our quality experts for a deeper look into our QMS software.
We will discuss the following topics in more detail:
- What Is the Definition of an SOP in the Pharmaceutical Industry?
- Why Are SOPs Important in the Pharmaceutical Industry?
- What Are the Different Types of SOPs in the Pharmaceutical Industry?
- What Are the SOP Management Guidelines in the Pharmaceutical Industry?
- What Is an SOP Format?
- What Are the SOP Management Process Steps?
- What Are the Best Practices for Writing an SOP in the Pharmaceutical Industry?
- What Is the Role of QMS Software for SOP Management?
What Is the Definition of an SOP in the Pharmaceutical Industry?
The SOP in the pharmaceutical industry is a validated and documented method that serves as the base of process activities, offering personnel clear step-by-step guidance for executing specific tasks to ensure compliance of company processes to internal policies and regulatory standards.
An SOP is used to streamline the pharmaceutical Quality Management System (QMS) processes, contributing to uniform and high product quality and safety.
Different regulations and standards are applicable depending on the market in which companies operate. These requirements governed by specific regulatory agencies have their definition for an SOP.
Below are more definitions of an SOP according to some of the main regulatory bodies.
Food and Drug Administration (FDA)
The FDA 21 CFR Part 211 defines SOPs as written procedures describing how to perform specific tasks or operations in producing pharmaceutical products.
They are designed to ensure drug products’ identity, strength, quality, and purity.
European Medicines Agency (EMA)
For EMA, the SOP refers to organized and easily verifiable instructions for operations.
According to EudraLex Volume 4 Good Manufacturing Practices (GMP) Chapter 4, they should be written in an imperative mandatory style.
International Organization for Standardization (ISO)
ISO specifies procedures as ways to carry out activities or processes.
They ensure that work is done consistently and to the required quality standards, as per ISO 9001:2015 for quality management systems.
World Health Organization (WHO)
According to the WHO guide to GMP requirements, SOPs are authorized written procedures providing general instructions for various operations, such as equipment operation, maintenance, cleaning, validation, and inspection.
What Is the Difference Between an SOP and a Work Instruction?
A standard operating procedure (SOP) is a document that provides high-level guidelines for a process. A work instruction is a more detailed step-by-step guidance document that provides accurate instructions on how to perform a particular task within that process.
Why Are SOPs Important in the Pharmaceutical Industry?
The SOPs are important in the pharmaceutical industry because they ensure that drug products are manufactured and handled in a consistent and controlled manner. This helps to reduce the risk of quality issues and noncompliances.
The main objective of an SOP is to ensure that tasks are performed consistently, correctly, and to the required quality standards, which can lead to several benefits for pharmaceutical companies.
Here are some reasons why SOPs are so important in the pharmaceutical industry.
Compliance With Regulations
The pharmaceutical industry is highly regulated, and SOPs help companies to comply with relevant requirements by ensuring consistent performance. This is important since noncompliance can result in monetary penalties, recalls, or legal action.
Ensure Quality and Safety
SOPs help ensure the quality and safety of pharmaceutical products by providing clear instructions on performing tasks. A comprehensive understanding of the procedure steps helps to reduce the risk of quality errors and contamination.
Improve Efficiency
Process efficiency in the pharmaceutical industry is improved by employing SOPs that streamline workflows and eliminate unnecessary steps. This can lead to increased productivity and reduced costs.
Transfer Knowledge
Procedures help to transfer knowledge within the pharmaceutical company by providing a way to document processes. SOPs are especially helpful for training new employees or moving employees between departments.
The SOPs ensure that a wide range of activities are performed correctly and effectively. Specific SOPs are tailored for each type of activity, ensuring precise steps are followed for each process.
What Are the Different Types of SOPs in the Pharmaceutical Industry?
There are different types of SOPs in the pharmaceutical industry. The SOP type varies depending on the departments, roles, and responsibilities.
The following are some examples of the most common types of SOPs in the pharmaceutical industry:
- Training SOP: Outlines training procedures, ensuring employees have the necessary knowledge and skills to perform their tasks.
- Safety SOP: Specifies the steps involved in ensuring the safety of employees and drug products. This includes procedures for handling hazardous materials, as well as procedures for responding to accidents and emergencies.
- Product Distribution SOP: Governs the distribution and transportation of pharmaceutical products. It ensures proper handling, storage, and documentation throughout the supply chain to maintain product integrity and prevent deviations during distribution.
- Cleaning SOP: Provides instructions for cleaning and sanitization processes within the pharmaceutical facility. It details cleaning agents, frequencies, and actions to maintain a sterile or non-sterile environment and prevent contamination.
- Production SOP: Covers the step-by-step procedures for drug manufacturing. It ensures consistency and compliance with GMP requirements, specifying the processes from raw materials to the finished product.
- Equipment Maintenance SOP: Outlines the steps in maintaining and calibrating critical equipment. This includes procedures for inspecting, testing, and repairing equipment.
- Quality Control SOP: Defines the steps involved in testing pharmaceutical products to ensure they meet established quality standards and specifications. This includes procedures for sampling, testing, and analyzing products.
In the pharmaceutical industry, there are different types of SOPs for each specific action that needs to be taken. The requirements for these processes are outlined by various guidelines, depending on the market in which the companies operate.
SimplerQMS provides a comprehensive eQMS that enables companies to store and manage various types of SOPs.
Our software utilizes secure cloud infrastructure to store all SOP-related documents and data. SOPs are accessible from anywhere and at any time. Team members can access documents and collaborate efficiently, whether in the office or remotely.
What Are the SOP Management Guidelines in the Pharmaceutical Industry?
In the pharmaceutical industry, SOP management guidelines encompass the regulations, standards, and requirements that dictate the creation, implementation, and maintenance of SOPs.
Below are some key guidelines applicable to the pharmaceutical industry.
NOTE
This section will discuss some guidelines applicable to the management of SOPs in the pharmaceutical industry. However, this is not an exhaustive list. Please always refer to the official requirements applicable to your company.
FDA 21 CFR Part 211
Regulation 21 CFR Part 211 establishes the current Good Manufacturing Practice (cGMP) requirements for finished pharmaceutical products.
It requires pharmaceutical companies to have written SOPs for all aspects of drug manufacturing, including production, testing, and packaging.
Drug manufacturers must create and follow written procedures for production and process control to ensure their drug products have the intended identity, strength, quality, and purity, according to 21 CFR 211.100.
EudraLex Volume 4 GMP
The EudraLex Volume 4 GMP contains guidance for interpreting the principles of GMP for manufacturers of medicinal products for human and veterinary use within the EU member states.
It requires companies to have written SOPs for all aspects of pharmaceutical production, from quality control to documentation and personnel training.
Documents containing instructions should be approved, signed, dated, and organized in a clear and easy-to-understand way by authorized persons, as indicated in EU GMP Chapter 4 Section 4.3.
ICH Q7
The ICH Q7 provides guidelines and recommendations for the GMP requirements specific to manufacturing Active Pharmaceutical Ingredients (APIs).
Written procedures should be established for several processes, such as equipment maintenance, batch production, customer complaints, change control, and more.
SOPs related to the manufacture of APIs should be prepared, reviewed, approved, and distributed utilizing version control as per ICH Q7 Section 6.11.
WHO GMP Guideline
The WHO GMP Guideline is a framework to aid manufacturers in assessing their planned or existing documents describing their production methods.
It lists different types of SOPs as required documents for demonstrating compliance. The list includes SOPs related to facilities, equipment, production, quality control, quality assurance, and more.
The WHO GMP Guideline Section 6 states that quality assurance procedures should be in place to ensure that SOPs are enforced and properly used.
The specific guidelines that would apply to your pharmaceutical company will vary depending on the applicable regulatory requirements. It is important to know the applicable requirements to determine the necessary SOP types and their formats.
What Is an SOP Format?
An SOP format is a template used to write effective standard operating procedures.
There is no single SOP format that all pharmaceutical companies use. However, some common elements are found in most SOPs.
The SimplerQMS software solution allows pharmaceutical companies to create different types of SOPs using highly customizable templates. Companies can easily use their existing SOP templates inside the system and draft documents to their specific needs.
SOP documents can then be sent through an automated workflow for authoring, review, and approval.
Here is an example of a common format of SOP:
Header
The header includes essential information about the SOP, such as the title, SOP number, version, and effective date.
The information on the header helps identify the SOP and keeps track of its version number.
Purpose
The purpose section states the reason for creating the SOP.
This section defines the specific goals, objectives, or outcomes the procedure aims to achieve.
Scope
The scope outlines the boundaries and limitations of the SOP.
A well-described scope clarifies what the procedure covers and specifies the areas it does not address.
References and Related Documents
This section lists any external documents, regulations, or standards mentioned in the SOP.
A list of references provides sources for additional information and for demonstrating compliance with specific requirements.
Roles and Responsibilities
The roles and responsibilities section defines the individuals or job roles involved in executing the specific processes.
This part of the SOP clarifies who is responsible for each step or task.
Procedure
The procedure section is the core part of the SOP. It provides a comprehensive set of instructions for carrying out the process.
Each step should be clear, concise, and in chronological order.
Using active voice, bullet points instead of paragraphs, and short sentences are recommended.
Appendices
Appendices contain supplementary information, such as forms, templates, or supporting documents that complement the written standard operating procedure.
Revision History
The revision history lists the changes made to the SOP over time, including the version number, revision date, and a summary of the modifications.
This section helps ensure that employees are working with the most up-to-date SOP version. Providing an overview of how the SOP has developed over time and why specific changes were made.
Signatures
The signatures section contains the names, dates, times, and signatures of authorized individuals who have reviewed and approved the SOP. It ensures accountability and authorization.
Typical signatures in an SOP include:
- Prepared by: The signature of the person responsible for creating the SOP.
- Reviewed by: The signature of the individual who reviewed the SOP.
- Approved by: The signature of the individual who approved the SOP.
- Authorized by: The personnel’s signature granting permission for the SOP’s implementation. Usually, departmental heads approve SOP implementation.
These SOP elements collectively create a well-structured and comprehensive SOP, providing clear guidance for performing specific activities consistently and effectively. Having a complete SOP format facilitates access to information and a more streamlined SOP management process.
What Are the SOP Management Process Steps?
The SOP management process steps involve a series of actions aimed at creating, implementing, and maintaining standard operating procedures.
The typical SOP management process steps are listed below.
- Preparation
- Review
- Approval
- Implementation
- Training
- Maintenance
A brief overview of each process step is provided below, with examples of how these steps are streamlined using an eQMS.
1. Preparation
The first step in the SOP management process is to prepare for the creation of an SOP.
This involves identifying the need for an SOP, gathering information about the task that the SOP will describe, and developing a draft procedure.
The specific people who prepare SOPs in a pharmaceutical company will vary depending on the size and complexity of the company. Usually, an SOP writer is an individual who either performs the task or person that is responsible for leading the people doing the job.
SimplerQMS provides eQMS software with document templates to simplify the preparation of new SOPs. Companies can create different templates for specific types of SOPs using our customizable templates or migrate their own SOPs into the system.
2. Review
Once the draft has been developed, it needs to be reviewed by the appropriate people.
This includes the person responsible for implementing the SOP and any other personnel who may be affected by the SOP.
Within SimplerQMS, assigning responsible people for document review is simple. You can assign reviewers by selecting relevant people from a dropdown when creating the SOP.
Automated notifications and reminders are sent to inform users about their tasks. This helps prevent delays and ensures that responsibilities are promptly addressed.
3. Approval
After the review process, the SOP is approved by authorized personnel who have the authority to sign off on the procedure. This approval signifies that the SOP is ready for use.
For example, SimplerQMS allows you to route the document to the pre-defined approver(s) in the workflow. The SOP is approved using electronic signatures with unique username and password combinations.
Each change in the SOP status is registered in the time-stamped audit trail, providing a comprehensive record of actions taken and the users involved.
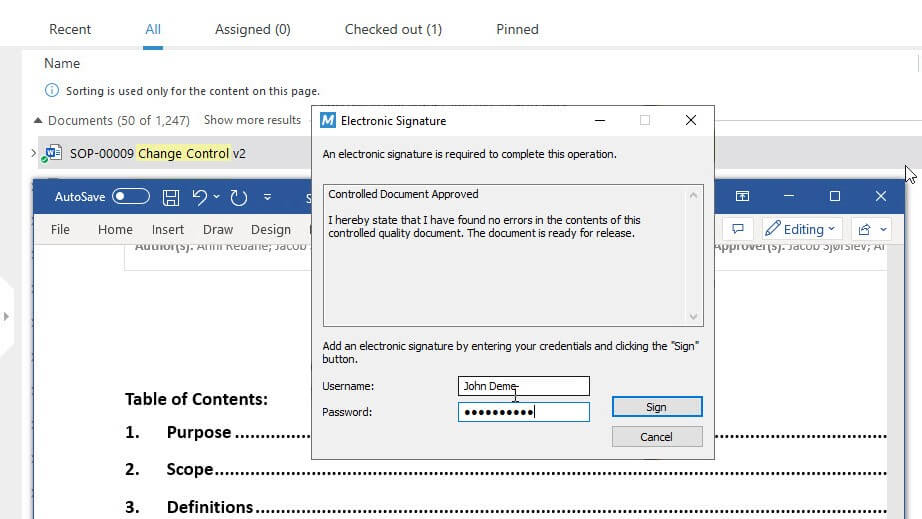
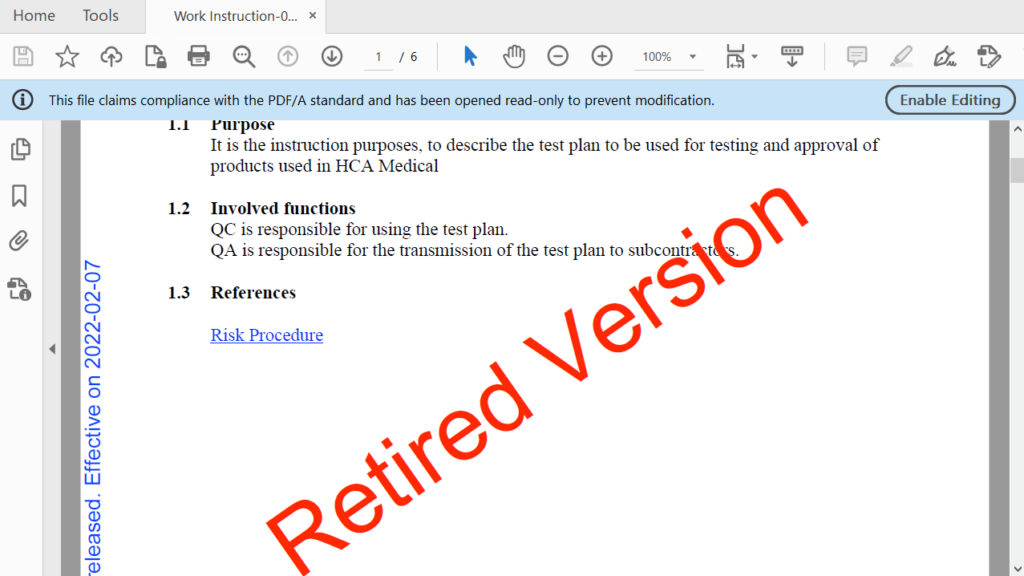
4. Implementation
Once approved, the SOP is implemented and put into practice.
After implementation, the SOP becomes an official document that guides employees in performing the specified tasks or processes.
In SimplerQMS, once an SOP update is implemented, the previous version of the SOP is retired to prevent the use of outdated procedures. New document versions are controlled using the software’s version control capabilities.
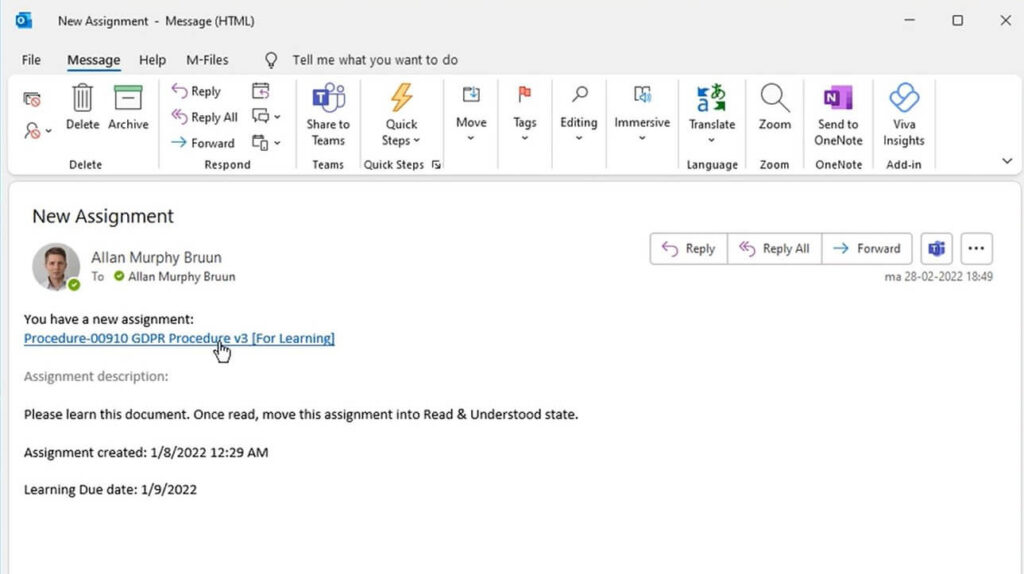
5. Training
Employees are trained on the SOP to ensure they understand the procedures and can follow them correctly. Training is essential to ensure process consistency and compliance with requirements.
To streamline this process, specific learning rules can be created in SimplerQMS to automatically send new or updated SOPs to all relevant people for training.
Training assignments in the system can include various materials for training, such as training videos, documents to learn, quizzes to complete, and more. All training records are stored in the system so that training can be tracked easily.
6. Maintenance
SOPs are living documents and may require updates over time due to changes in regulations, processes, or best practices. Regular maintenance and revision of SOPs help keep them up-to-date and relevant.
In SimplerQMS, users can define periodic review tasks for each SOP, specifying how often the document should undergo a review. As the SOP review date approaches, the software automatically notifies the designated reviewers or approvers, prompting them to do the review task.
To further improve user-friendliness and effectiveness, SOPs should be written with clarity and precision, employing best practices for SOP writing.
What Are the Best Practices for Writing an SOP in the Pharmaceutical Industry?
To maintain clarity and accuracy in standard operating procedures, it is essential to follow best practices for SOP writing.
Consider the following best practices for developing pharmaceutical SOPs:
Have a Clear and Concise Scope
The scope of an SOP should be clearly defined at the beginning of the document. This will help ensure that the SOP only covers the specific procedure or process it is intended for.
Use Plain Language
SOPs should be written in plain language that is easy to understand by anyone who needs to use them. Avoid using jargon or technical terms that the reader may not be familiar with.
Be Specific
The SOPs should be as specific as possible. Provide detailed and specific instructions for each step to leave no room for interpretation or ambiguity.
Use Diagrams and Illustrations
Incorporate visual aids like illustrations, flowcharts, and diagrams to supplement the textual descriptions, making complex processes easier to comprehend.
Get Input From Experts
Involve subject matter experts to validate the accuracy and completeness of the SOP, ensuring it reflects the best industry practices.
Review and Update the SOP Regularly
SOPs should be reviewed and updated regularly to ensure they are accurate and up-to-date. This is especially important if there are any changes to the procedures or processes they cover.
By following these best practices, pharmaceutical companies can develop SOPs that help ensure consistent process execution. The whole process can be further improved by implementing QMS software to streamline the process.
What Is the Role of QMS Software for SOP Management?
QMS software plays a crucial role in SOP management by facilitating the creation, maintenance, and control of standard operating procedures within a company. It streamlines the entire SOP lifecycle, from creation to approval to implementation and maintenance.
In some companies, the management of SOPs can be accomplished through paper-based or hybrid systems, which depend on company size and available resources.
However, these systems come with certain drawbacks, as they can be time-consuming and labor-intensive. Challenges may arise in tracking changes, potential document loss, the need for physical storage space, and other related issues.
A better alternative is an electronic Quality Management System (eQMS) software, which offers powerful SOP management capabilities.
SimplerQMS provides eQMS solutions specifically designed for Life Science companies.
With SimplerQMS, companies can benefit from features such as automated workflows, version control, and time-stamped audit trails.
We offer QMS software with robust document management capabilities that simplify the SOP creation, review, approval, and update process. The system is cloud-based and limits access to authorized personnel, ensuring that documents are secure.
The software promotes efficient collaboration among team members, as they can easily find and retrieve the SOPs they need using a search function. Controlled printing capability allows for managing the print or download of copies of SOPs and facilitates keeping track of all printouts.
Our eQMS platform helps companies achieve compliance with several Life Science requirements, including ISO 9001:2015, ISO 13485:2016, FDA 21 CFR Part 11, 210, 211, and 820, EU GMP Annex 11, EU GMP, and more. The SimplerQMS solution supports companies in regard to compliance with regulatory requirements by providing comprehensive QMS process support.
The SimplerQMS solution supports several QMS processes, including document management, change control, audit management, employee training management, CAPA management, customer complaint management, supplier management, and more.
Discover the benefits of implementing an eQMS in your company with our eQMS Business Case template. Identify opportunities for efficiency, cost reduction, and improved compliance. Assess the advantages of an eQMS and present essential findings to management.
Final Thoughts
Effective SOP management is essential to ensure that SOPs promote consistency, standardization, and efficiency in the execution of processes within a company.
The core purpose of Standard Operating Procedures (SOPs) is to provide a comprehensive guide on how processes and routine operations are carried out by the company.
Although SOP management can be done using paper-based or hybrid systems, these methods often encounter issues such as lost documents and increased error-prone processes.
To address these challenges, companies utilize eQMS to manage SOPs. eQMS software, like SimplerQMS, provides robust document management features that streamline the creation, control, and archiving of SOPs.
With SimplerQMS, pharmaceutical companies gain access to SOP templates, automated workflows for process steps, document version control, and more, facilitating efficient and streamlined SOP management.
If you are interested in learning more about how SimplerQMS can help you streamline your quality management activities, we recommend you book a tailored demo today and talk to our system experts.