ISO 13485 QMS Software
Simplify ISO 13485 compliance with QMS software designed to help you streamline processes and support regulatory adherence.
TRUSTED BY
























Compliant ISO 13485 Software for Medical Device QMS
SimplerQMS offers ISO 13485-compliant software for complete QMS management, providing extensive process support.
Medical device companies implement the ISO 13485-compliant Quality Management System to demonstrate that they consistently meet customer and regulatory requirements.
Our QMS software is built on the ISO 13485 QMS framework. It supports processes such as document management, change control, training management, design control, risk, supplier management, nonconformance, CAPA management, and more.
Our platform helps companies comply with various Life Science requirements, including ISO 13485 as well as FDA 21 CFR Part 11, 210, 211, 820, EU MDR & IVDR, GxP, ICH Q10, ISO 9001, EU Annex 11, and others.
Optimize Your QMS Processes With Interconnectivity
SimplerQMS provides interconnected QMS modules, such as document control, change control, training, supplier, nonconformance, CAPA, audit management, and others.
Link related documents, assignments, and records with ease and facilitate easy access to related information.
Improve overall efficiency and productivity with an interlinked QMS system that ensures traceability, simplifies audits and promotes a seamless flow of information.
Work on Documents With Microsoft Office Integration
SimplerQMS allows you to edit, review, and approve documents effortlessly within Microsoft Office applications such as Word, Excel, and PowerPoint.
Streamline the document creation and editing processes. Save documents inside SimplerQMS with one click and eliminate the need for repetitive downloads and uploads.
Enjoy a seamless workflow that empowers your team to work together – collaborate on documents and accelerate document turnaround time.
Implement a Fully Validated System
Computer software used in the quality management system must be validated according to ISO 13485:2016 section 4.1.6. SimplerQMS offers a fully validated eQMS according to ISPE GAMP5.
With SimplerQMS, you can save valuable time and resources by avoiding software validation – we take care of the whole process.
We handle all aspects of software validation and revalidation so that you can focus on core business activities.
Utilize Our Forms and Templates and Simplify Their Management
SimplerQMS provides a complementary form and template package based on ISO 13485:2016 requirements to kickstart your quality management system.
You can also use your own forms and templates if necessary.
With SimplerQMS you can centralize the management of forms and templates within one system, and ensure easy access and control of all your forms and templates.
Ensure Regulatory Compliance
Our software is built on the ISO 13485 framework to support essential QMS processes as outlined by the regulatory standard.
SimplerQMS goes beyond the support for ISO 13485 requirements and helps companies achieve compliance with other life science requirements, such as GxP, EU MDR and IVDR, FDA 21 CFR Part 11, 210, 211, 820, EU GMP Annex 11, ICH Q10, and many others.
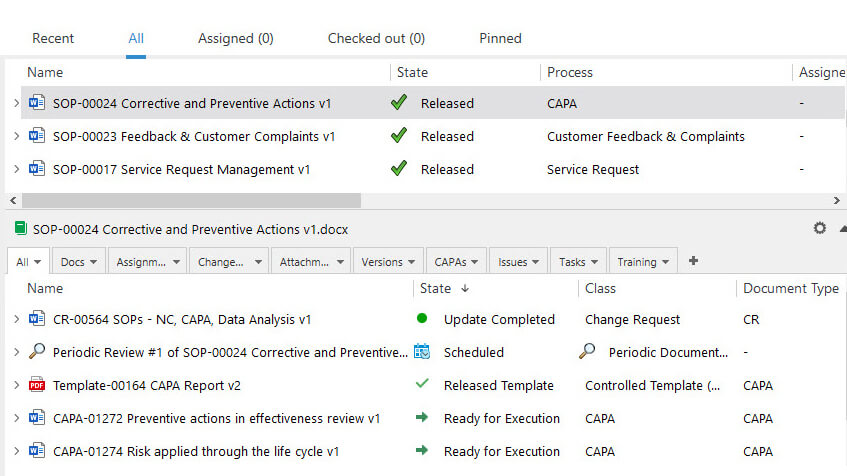
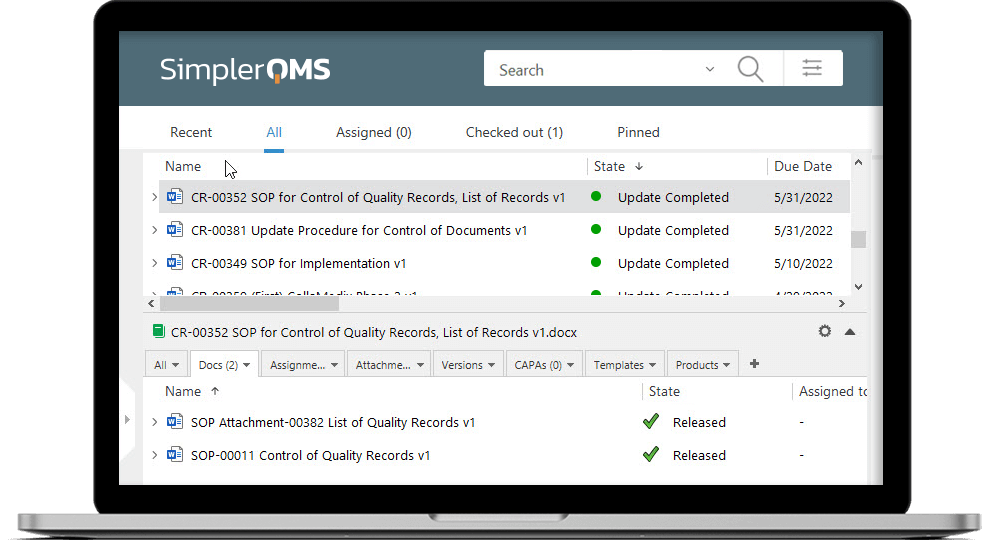
Achieve Control With Document and Change Controls
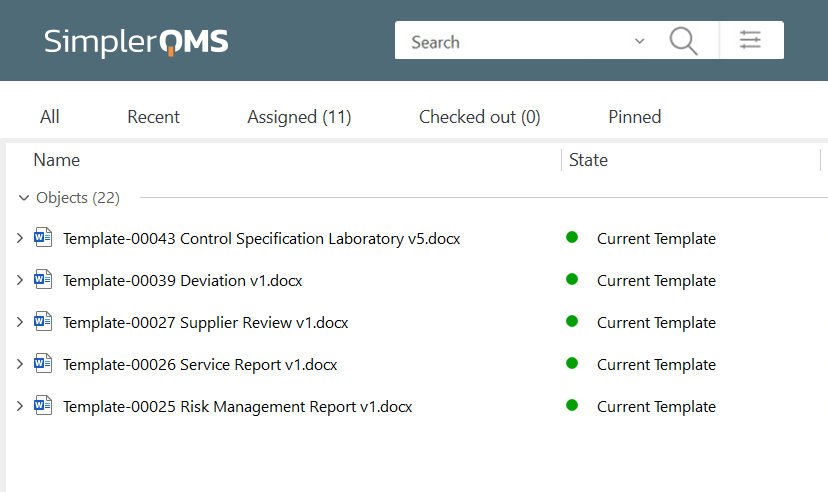
Control of QMS documentation is a requirement according to ISO 13458:2016 section 4.2.4. SimplerQMS provides robust document control capabilities to streamline document control and management. Users can store all documents in a single and secure location within the SimplerQMS cloud. Ensure controlled access and simplified document retrieval.
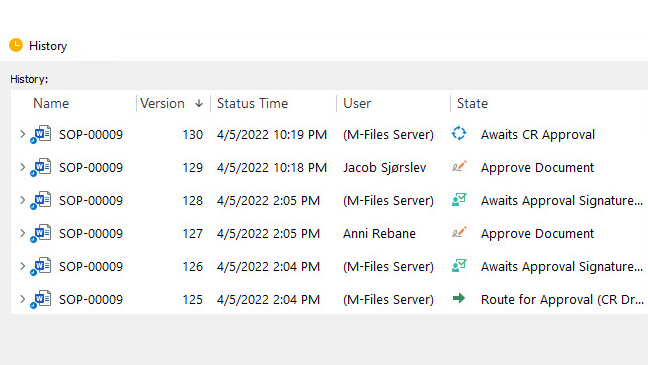
Keep track of document versions and changes effortlessly with a detailed version history log. Easily navigate through different document versions, view modifications, and roll back to the previous version if necessary. Furthermore, SimplerQMS helps streamline change control management processes and easily initiate, track, and control changes.
Streamline Training Management for Efficient Employee Development
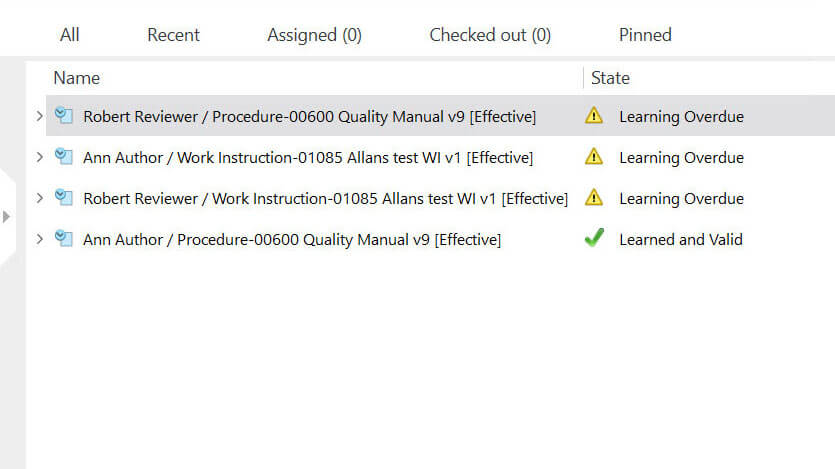
Medical device companies must provide training to achieve and maintain the necessary competence among employees as per ISO 13485:2016 section 6.2. SimplerQMS simplifies the management of employee training and certifications with automated workflows built into its training management module. You can streamline the entire training process, from initial onboarding to ongoing professional development.
Create, schedule, and track training assignments with automated reminders and notifications to ensure training completion. Maintain accurate records of completed training and certifications, ensuring compliance with regulatory requirements.
Proactively Mitigate Risks With Integrated Risk Management
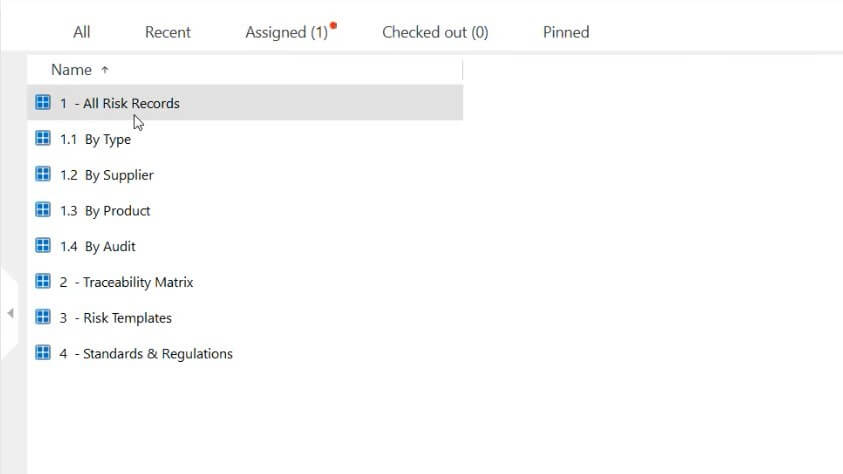
Medical device companies must have a risk-based approach to control processes related to the quality management system as specified in ISO 13485:2016 section 4.1.2. With the SimplerQMS risk management module, you can effectively manage your risk management documentation.
Integrate risk management into other quality processes and make informed decisions based on potential risks. Simplify handling risk management files to record and track all risk-related information. Easily create, edit, and retrieve risk files in the system.
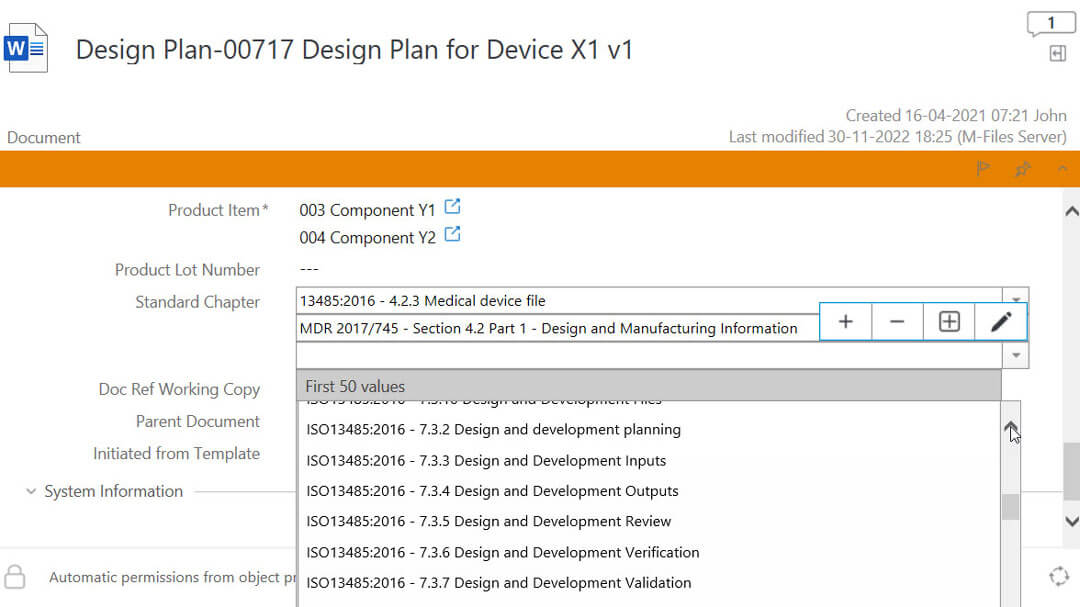
Expedite Design Control Documentation
It is necessary to document procedures for the stages of design control, from initial realization to final product release, following ISO 13485:2016 section 7.3.
SimplerQMS allows you to streamline design control processes for medical device development.
Centralize all relevant documents, such as design inputs and outputs, verification and validation records, and design reviews. Prepare for regulatory submissions by utilizing document collections. Assemble submission-ready documentation and streamline the submission process.
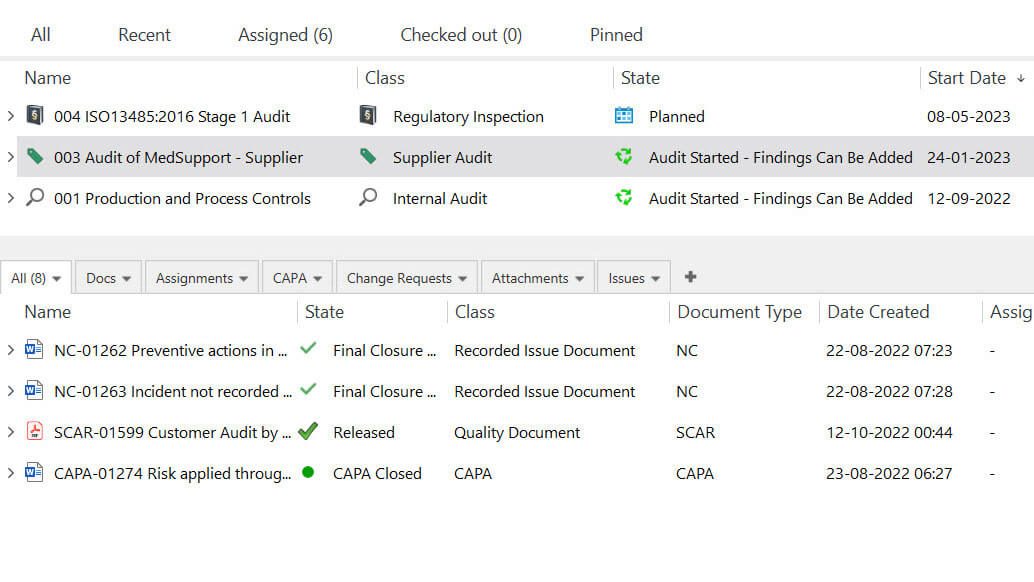
Streamline Audits With Effective Audit Management
With the SimplerQMS audit management module, you can plan audits, assign tasks, and track the progress of audit activities.
Streamline audit processes (for internal audits, regulatory inspections, and supplier audits) by utilizing a centralized repository for all information.
Simplify document retrieval and ensure all necessary documentation is readily available for internal and external audits and inspections.
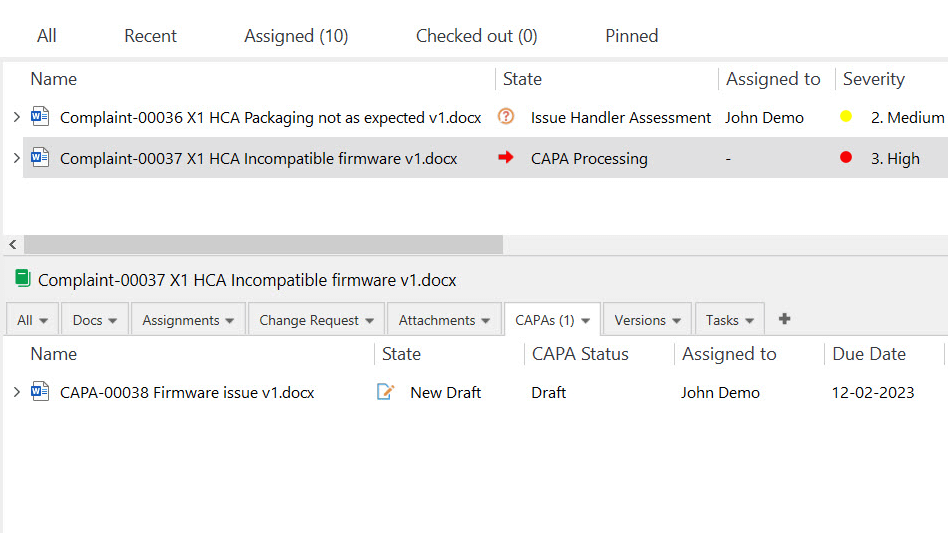
Resolve Customer Complaints Promptly
Customer complaints must be handled in a timely manner and properly documented as outlined in the ISO 13485:2016 section 8.2.2.
SimplerQMS allows you to efficiently handle customer complaints and feedback and ensure prompt and valuable responses to customer concerns.
Track customer complaints and feedback, gain insights into recurring issues and identify areas for improvement. Investigate customer complaints thoroughly and initiate appropriate corrective and preventive actions (CAPA) to address the underlying issues if necessary.
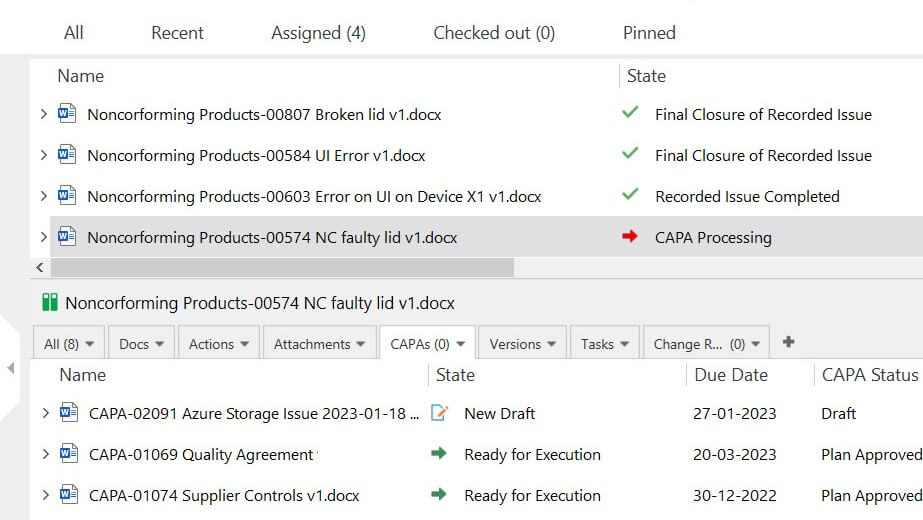
Manage Nonconfomances Effectively
Medical device companies must identify and control nonconformances to prevent devices’ unintended use or delivery as outlined in ISO 13485:2016 section 8.3.
With the SimplerQMS nonconformance management module, you can handle nonconformances with ease and keep track of all actions and statuses related to issues via customizable views.
Easily escalate nonconformances to corrective and preventive actions (CAPA) if necessary.
Make sure that all relevant documents are available to responsible people. Simplify assigning of responsibilities, defining timelines, notifying relevant personnel, and monitoring progress.
Implement Effective CAPA Management Process
Companies must implement corrective and preventive action (CAPA) processes to eliminate the causes of potential nonconformances and prevent their occurrence in accordance with ISO 13485:2016 sections 8.5.2 and 8.5.3.
SimplerQMS provides a closed-loop CAPA management workflow for you to seamlessly manage such issues.
Track and resolve quality events effectively to ensure resolution in a timely manner. Monitor the progress of CAPA activities using customizable views. Implement measures to prevent the recurrence of similar problems in the future – set actions to assess their effectiveness.
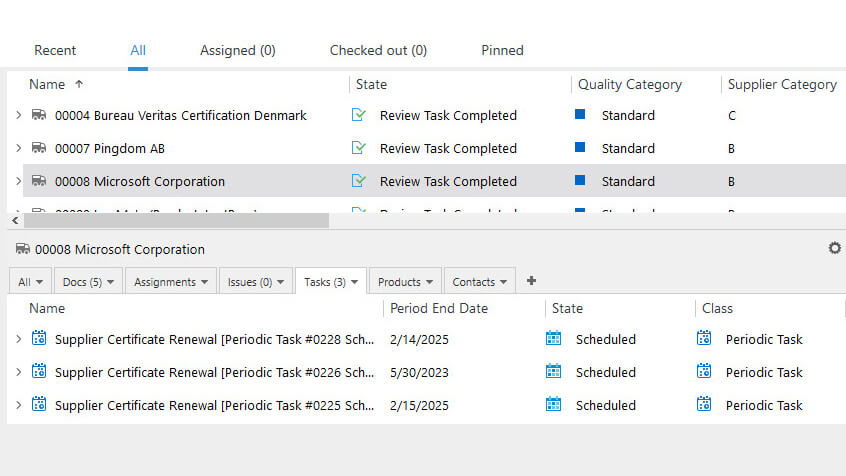
Optimize Supplier Management Processes
SimplerQMS simplifies medical device supplier management processes and helps ensure compliance with the requirements outlined in ISO 13485:2016 section 7.4.1.
Perform supplier qualification based on established criteria and define the supplier category, type, related product, and associated risks. Monitor supplier performance by scheduling audits, assignments, and periodic review tasks.
Create and maintain approved supplier lists (ASL), and relate and access documents such as suppliers’ contracts, evaluations, and certificates with ease.
Transparent Pricing Structure
SimplerQMS provides all QMS modules and features for one price. The total cost of the subscription is determined based on the type and quantity of user licenses required.
We offer flexibility in pricing plans, allowing you to choose the most suitable option for your company.
There are NO extra fees for additional modules, implementation, validation, or training. Everything you need is covered within the subscription price, ensuring straightforward and transparent pricing.
What Customers Achieve By Implementing SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audit More Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Complete eQMS for ISO 13485 Compliance
Change Management
Establish a robust change management process encompassing proper review, approval, and communication of all document changes.
Training Management
Develop comprehensive training plans, automate notifications, and track progress to ensure efficient and effective employee training.
Audit Management
Improve the efficiency and reduce the time required for audit management processes.
Supplier Management
Optimize and streamline all supplier-related activities, ensuring the high quality of products and services obtained from suppliers.
Nonconformances
Effectively identify, manage, and resolve nonconformances in compliance with regulatory requirements.
CAPA Management
Implement preventive and corrective actions and prevent the recurrence of recorded issues.
Frequently Asked Questions
What Is a 13485 QMS Software?
ISO 13485 QMS software refers to a specialized computer program designed to help medical device companies manage their quality management processes electronically and in accordance with the ISO 13485:2016 standard.
What Makes an ISO 13485 Software Compliant?
ISO 13485-compliant software is a software solution that follows the medical device QMS framework outlined in the ISO 13485 regulatory standard.
It provides the necessary QMS modules and features to support implementing and maintaining a QMS compliant with ISO 13485. With this software, companies can streamline processes such as document management, risk management, change control, CAPA, audit management, training, and others.
Why Is ISO 13485 Quality System Software Important?
The ISO 13485 Quality System software is important because it helps medical device companies follow the quality management processes to achieve compliance with ISO 1348, simplify documentation processes, and enhance collaboration and efficiency.
Who Needs to Use an ISO 13485 Compliant QMS Software?
Using ISO 13485 QMS software is beneficial for any company involved in one or more stages of the life cycle of a medical device, including the design, development, manufacturing, or distribution, among others.
This includes medical device manufacturers, contract manufacturers, suppliers, distributors, and companies providing services such as calibration, maintenance, or repair.
By using ISO 13485-compliant QMS software, companies can streamline their quality management processes and make regulatory compliance easier. This can help them improve overall efficiency, gain market access, and demonstrate a commitment to high and uniform quality and safety.
How Much Does ISO 13485 QMS Software Cost?
The total cost of the SimplerQMS solution depends on the type and number of licenses chosen.
With SimplerQMS, you get a comprehensive solution that includes all QMS modules, hosting, validation, implementation, user training, and ongoing support. All for a single subscription price.
This means there are no extra fees to worry about.
For detailed information about SimplerQMS pricing and the features and services included, please visit our pricing page.
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To learn how you can make the most of SimplerQMS in your medical device company – book a tailored demo.