An effective quality management system (QMS) is a system that enables a company to consistently provide products and services that meet customer and regulatory requirements.
Effective quality management has become a necessity in the Life Science industry. A comprehensive QMS can often mean the difference between a company’s success and failure.
Ineffective quality processes may create risks for non-compliance, which could lead to a recall of products or other issues. Such issues are not only costly and time-consuming but can also damage the company’s reputation or slow down product development and further postpone market access.
This article will focus on how to measure the effectiveness of the Life Science company’s quality management processes. We will briefly describe what a QMS is, touch upon different QMS types, and explore how to measure the effectiveness of the various quality management processes.
One way to improve the effectiveness of your QMS is to implement a digital solution such as an Electronic Quality Management System (eQMS).
SimplerQMS provides a cloud-based QMS software solution designed for the Life Science industry. The system helps manage quality management processes and makes it easier to achieve regulatory compliance. If you want to learn more about how our software solution can help you, contact us for a demo.
In this article, you will learn the following:
- What is a Quality Management System (QMS)?
- Types of Quality Management Systems
- Processes of an Effective Quality Management System
- Benefits of Ensuring an Effective Quality Management System
- Improve Your Quality Management System by Using QMS Software
What is a Quality Management System (QMS)?
A Quality Management System (QMS) is the whole system that a company uses to control, organize and control documentation. It helps ensure its products’ high and uniform quality and mitigate risks for the end user.
A QMS can be paper-based, hybrid, which is part paper-based and part electronic, or entirely electronic.
A manual paper-based QMS is a traditional way of managing quality processes. This system requires employees to manually input, store and retrieve data using physical copies of the documents.
The hybrid QMS combines elements of paper-based and electronic systems. It allows companies to automate specific activities while keeping the manual work for other processes.
An Electronic Quality Management System (eQMS) like SimplerQMS enables companies to send automatic notifications, store documents in a cloud-based repository, automatically record all activities performed inside the system for audit trail, define access levels to documents, search and retrieve files easily, and more.
You can learn more about a QMS and its components in our Quality Management System (QMS) guide.
Types of Quality Management Systems
Each Life Science field has specific requirements that companies must comply with to ensure their products’ safety and efficacy.
As such, a QMS must be tailored to the specific product type, industry, and intended market.
Below are a few of the requirements related to QMS applicable to specific Life Science industries. It is advisable to always verify the relevant requirements in your area.
Medical Devices
For instance, companies that wish to place medical devices in the European Union market must adhere to the EU regulation 2017/745, also known as Medical Device Regulation (MDR). In this case, the medical device QMS must comply with the relevant requirements specified in the MDR.
You can read our article EU MDR Quality Management System to learn more.
On the other hand, in the United States, the relevant regulation for medical devices QMS is the FDA 21 CFR Part 820.
You can find more information about the 21 CFR Part 820 quality system regulation here.
The ISO 13485:2016 is an international regulatory standard that specifies the requirements for Quality Management Systems (QMS) in the medical device industry.
Certain countries accept ISO 13485:2016 certification as proof of conformity regarding QMS, which can speed up national inspection in some cases, such as MDR. You can learn more by reading the article regarding ISO 13485:2016 compliant QMS.
Pharmaceutical
In regards to the pharmaceutical quality management system, there are several different requirements, such as Good Manufacturing Practices (GMP), Clinical (GCP), Laboratory (GLP), and more.
For instance, in the United States, the FDA 21 CFR Part 210 specifies the minimum current GMP (cGMP) for manufacturing, processing, packing, or holding a drug to ensure the product’s safety, quality, and purity characteristics. Furthermore, the cGMP requirement for finished pharmaceuticals is specified in the FDA 21 CFR Part 211 regulation.
Feel free to read our article if you are interested in learning how GMP compliance is reflected in QMS and the role of eQMS.
Another international standard for QMS requirements is the ISO 9001:2015. It is a general standard that applies to many industries and is also used by pharmaceutical companies.
Another important pharmaceutical QMS guideline is the ICH Q10 implemented in the ICH member countries, for example, European Union, the United States, Brazil, Japan, Canada, Mexico, the UK, and more.
You can find more information about the ICH Q10 pharmaceutical quality system model in our guide.
Medical Laboratories
In the medical laboratory industry, a very know international standard is ISO 15189:2022. It focuses on promoting patient welfare and satisfaction by instilling confidence in the quality and competence of medical laboratories. To learn more about what ISO 15189:2022 is, read our detailed introduction to ISO 15189:2022.
Another important standard is the ISO 17025:2017, which outlines the requirements for laboratories to prove they are capable of testing and calibrating equipment. By complying with this ISO standard, a facility can generate valid results through competence, impartiality, and consistency.
Medical laboratories in the United States are required to comply with the Clinical Laboratory Improvement Amendments (CLIA), which aim to ensure the accuracy and reliability of testing in all laboratories that perform testing on humans. The specific requirements for CLIA are outlined in regulation FDA 42 CFR Part 493.
Processes of an Effective Quality Management System
An effective quality management system comprises different quality processes that create a system to help ensure the quality and safety of products and services.
Management review is an evaluation of the effectiveness of the quality management system as a whole.
The management review process is a formal evaluation conducted periodically to assess the performance of the complete QMS. The primary goal of this review is to determine the adequacy, suitability, and effectiveness of the QMS for its intended purpose.
It involves reviewing and evaluating documents, processes, and procedures.
This section will list some processes that contribute to an effective QMS.
1. Document Management
Document management and document control are critical aspects of quality management systems involving creating, storing, retrieving, and managing documents.
It also helps ensure that documents are accurate and up-to-date and supports the company in achieving compliance.
Some of the main tasks of document management and control processes involve the following:
- Versioning documents and tracking changes
- Reviewing and approving documents
- Identifying changes to documents and keeping track of revisions
- Proper retention, protection, and archive of documents
Here are some ways to ensure the effectiveness of a document management process:
- Consistent document format: Templates can help establish a consistent format and style for documents and indicate where all the necessary information is located. The documents should include the author, reviewer, approver, effective date, and version number. Companies can utilize forms and templates to streamline document creation, ensuring document format consistency.
- Document description overview: A clear and concise overview of the document’s contents can be viewed in the metadata (when using an eQMS like SimplerQMS) or in the document header. This is important to identify all the relevant information regarding a document, such as an author, reviewer, approver, department, process, related documents, and applicable regulations.
- Periodic document review: Life Science companies must have documents with the correct content and all the relevant information, reflecting the current requirements and policies clearly and accurately. The review process involves systematically assessing documents to identify discrepancies or errors, determine if the documents are still current, and comply with requirements.
- Access to documents: Ensuring easy access is essential for relevant personnel to consult the documents when needed.
SimplerQMS helps Life Science companies to manage their documentation process by providing state-of-the-art document control software. Companies can effectively control documents, review documents for accuracy, make changes as necessary, and track actions.
By taking advantage of SimplerQMS pre-configured workflows and powerful features, you can ensure consistency and greater effectiveness in the documentation processes.
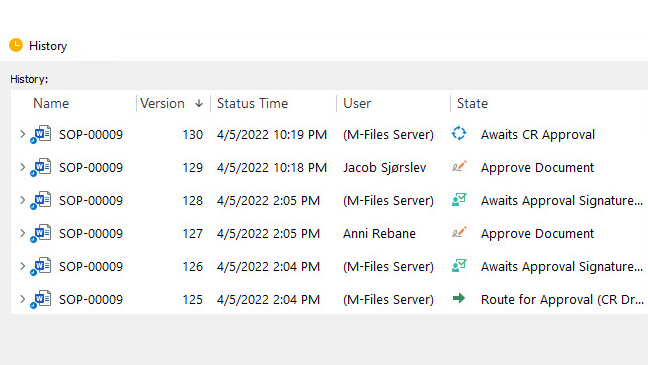
2. Change Management
Change management is an essential QMS process in the Life Science industry. It enables companies to control and document processes, procedures, and document changes.
If a change request modifies a process or a document, this needs to be communicated to relevant employees. It is also important to verify if the implications of this change are related to other documents and if a training session will be needed, among other things.
Effective change management requires the control of change requests, which are essential for ensuring that updates in relevant documents are evaluated and approved before implementing the change.
For example, to assess the efficacy of change management, companies may monitor metrics such as the number of open, closed, and overdue change requests, pending actions, and the time taken to complete a change request from start to finish. These metrics can help evaluate whether the average time to conclude a change request is acceptable.
SimplerQMS offers a change management software that controls all aspects of the change management process.
Starting from using best practice change request templates, specifying documents in scope for the change, routing changes for review and approval, and setting automatic reminders.
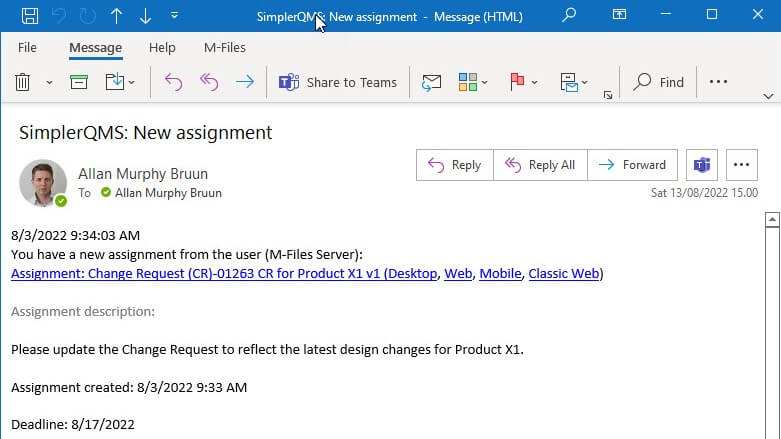
3. Training Management
A component of quality management is employee training management. This involves establishing and maintaining a systematic approach to ensuring that all personnel is adequately trained and competent to perform their roles effectively and safely.
Training management involves identifying the necessary training requirements, developing training plans and learning materials, providing the training, and documenting all aspects of the training process.
Effective training management can help companies in the Life Science industry to reduce errors, improve productivity, and improve overall quality performance.
Measuring the effectiveness of training management involves assessing if the training program has achieved its intended objectives.
Quizzes are a valuable tool for assessing training effectiveness. It can help to reinforce important concepts and ensure that trainees retain the information presented to them.
Quizzes can also help identify areas where trainees may be struggling and areas for improvement.
SimplerQMS provides a training management module to help Life Science companies streamline the training process. Training Managers can create learning rules and training groups, as well as assess training effectiveness with personalized quizzes.
It is easy to create and manage quizzes using our pre-configured workflows. You can use a multiple-choice format quiz and pre-defined passing criteria to monitor the performance of employees before and after the training.
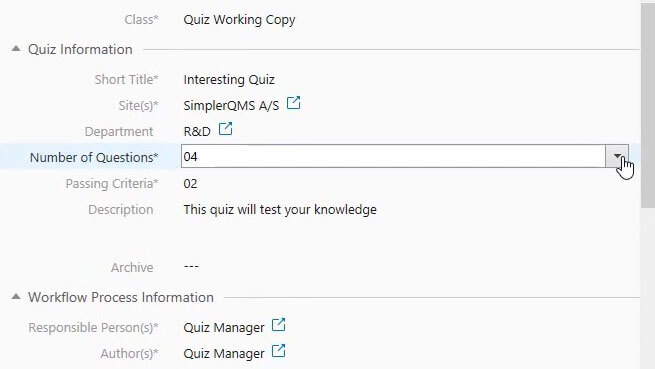
4. Customer Complaint Management
Having a process in place to handle complaints is a requirement for the Life Science industry. Moreover, customer complaints can help companies identify improvement areas and better understand customer requirements.
By managing the complaint process in the QMS, companies can comply with relevant requirements and demonstrate the intent to take action to ensure the safety and quality of their products.
For instance, in the medical device industry, the ISO 13485:2016 standard specifies that companies must gather and monitor information relating to whether the company has met customer requirements.
One essential step for measuring the effectiveness of complaint management is collecting data. Companies can gather data through various means, such as customer feedback forms and surveys.
With the data analysis, it is possible to monitor progress by evaluating your history of complaints to determine whether they are decreasing or increasing. It can also help identify trends and patterns, such as the number of critical complaints and the percentage of complaints that have escalated to a CAPA.
SimplerQMS solution streamlines the complaint-handling process so companies can quickly respond to customer issues.
Customizable views allow for a complete overview of all complaint statuses, including the document modification date and the person responsible for the action.
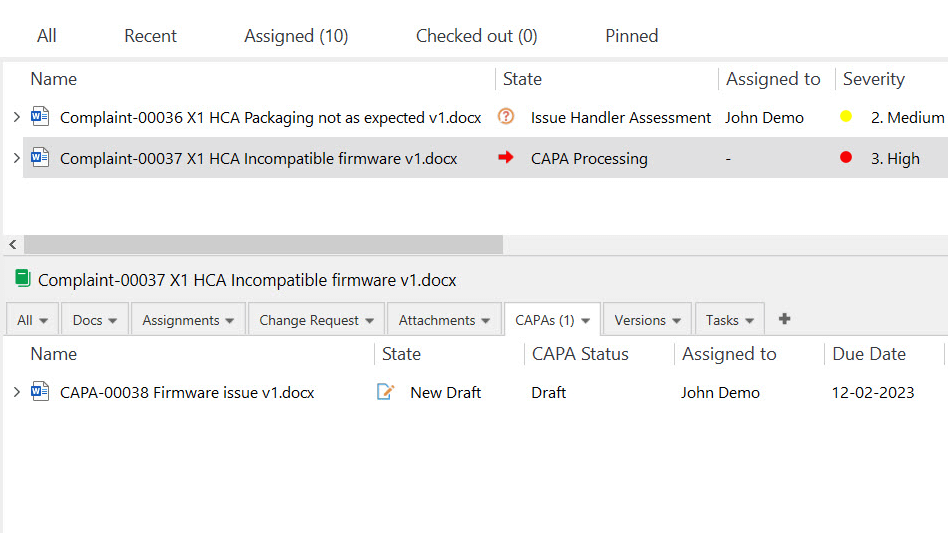
5. Nonconformance and Deviation Management
Nonconformance and deviation management processes are for identifying and managing events where a process or product does not conform as intended.
It involves documenting and investigating the nonconformance or deviations and implementing corrective and preventive actions if needed.
Measuring the effectiveness of the nonconformance and deviation process involves evaluating several metrics and trends.
One metric can be the number of issues identified and reported within a given time. It helps to identify areas where improvements may be necessary to reduce the occurrence of issues.
Another one is to evaluate if the nonconformances or deviations are being resolved in a timely manner.
Companies also perform effectiveness assessments and evaluate Key Performance Indicators (KPIs) to determine if the established processes had the intended result. It is useful to track the number of reopened nonconformances and deviation cases to measure if the corrective action was effective.
SimplerQMS nonconformance management or deviation management modules help Life Science companies to streamline the documenting, tracking, and resolution of nonconformances and deviations while promoting continuous improvement.
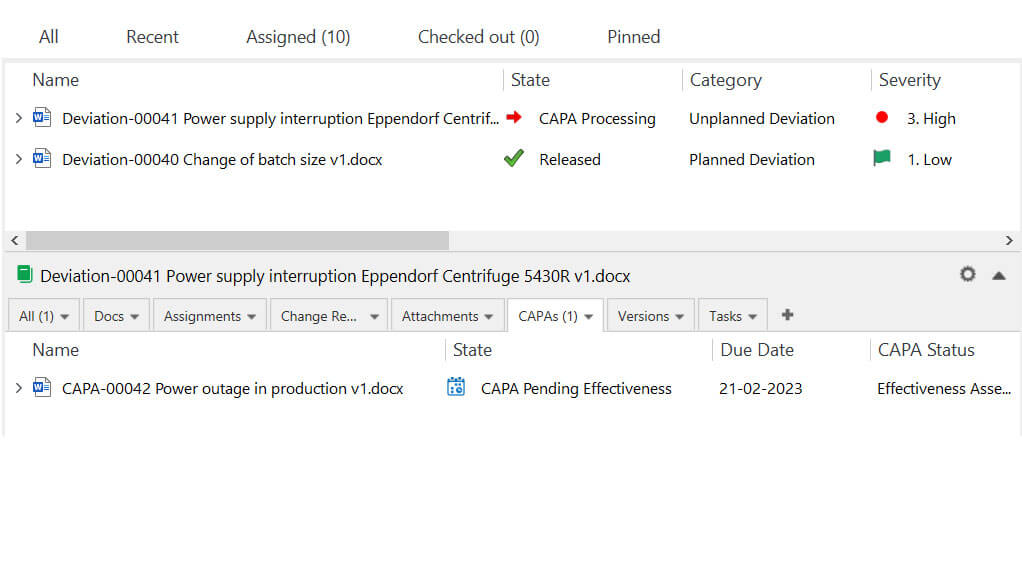
Furthermore, SimplerQMS facilitates data exports, allowing companies to analyze and monitor KPIs related to their quality management efforts.
6. CAPA Management
One way to handle issues and drive continuous improvement is by using a corrective and preventive action (CAPA) system. CAPA management is a process that helps companies identify and implement actions to correct quality issues and prevent them from reoccurring.
An effective QMS has an integrated CAPA process to help ensure issues are identified and addressed on time.
Assessing the outcomes of the implemented actions during scheduled effectiveness checks is essential in determining the effectiveness of the CAPA management process.
Another way is by monitoring metrics and trends associated with CAPA.
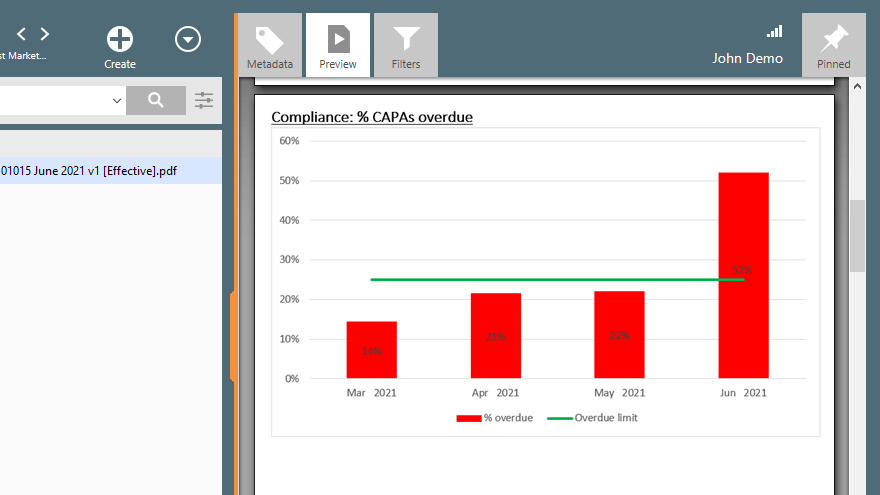
Companies can analyze data, such as the number of CAPAs opened, the time taken to close CAPAs, and the percentage of CAPAs that were closed in a timely manner. Those analyses can provide valuable insights into the effectiveness of the process.
SimplerQMS provides a complete QMS solution with an integrated CAPA management process to handle corrective and preventive actions. You can analyze CAPAs by issue type to find the most frequent problems related to products, components, customers, equipment, and suppliers.
You can also export all data related to CAPA for a more comprehensive data analysis, which provides valuable insights for improving the quality management system.
7. Audit Management
The audit management process aims to ensure the QMS works effectively, is aligned with the company’s quality objectives, and complies with regulatory requirements.
A requirement used to ensure continuous improvement is conducting audits. Audits help evaluate the effectiveness of the inspected processes. Companies must create periodic internal and external audit plans.
Supplier audits also help identify potential risks or issues with the supplier’s processes, which can be addressed and resolved before they affect the production process or product.
The effectiveness of the audit management process can be measured by evaluating if the audit process resulted in the identification and resolution of audit findings. It is also important to assess if the audit process has contributed to continuous improvement in quality performance.
Assessing the frequency and severity of audit findings can indicate whether the audit process effectively identifies quality issues.
Conducting trend analyses of audit findings over time can also help to determine if the audit process has led to sustained improvements in quality performance by avoiding reoccurring issues.
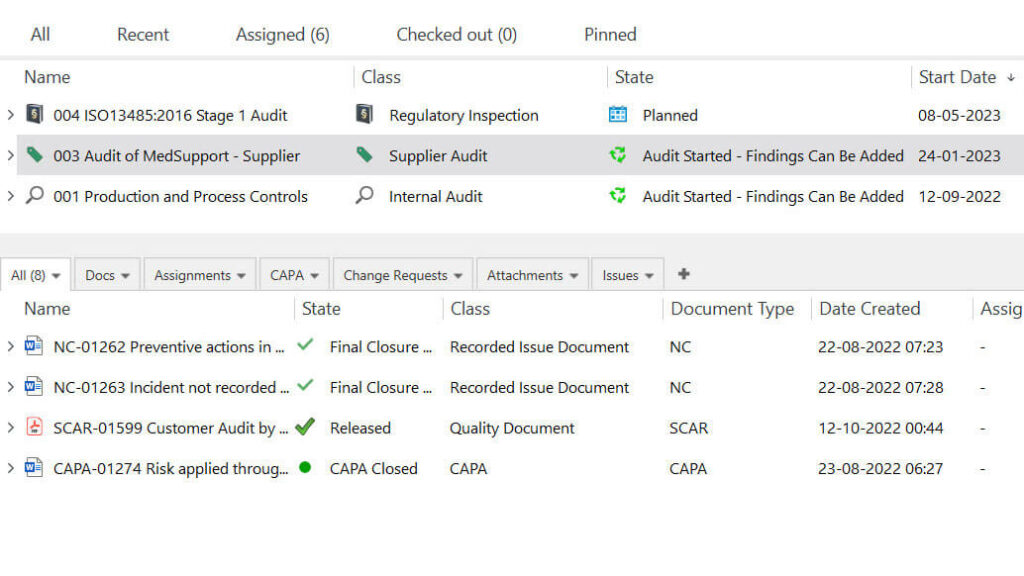
SimplerQMS offers life sciences companies audit management software for time-saving, efficient, and compliant audit processes.
You can easily schedule audits, assign responsible persons, attach evidence to audit findings and escalate to CAPA if necessary.
8. Supplier Management
The supplier management process helps Life Science companies ensure that the products or services that suppliers provide meet the company’s quality standards. It involves selecting and evaluating suppliers based on their ability to ensure quality, delivery, and other requirements.
Establishing an Approved Supplier List (ASL) can assist you by having a clear overview of all suppliers approved by a company. And having periodic reviews of the ASL ensures it is always up-to-date.
In a way, supplier audit findings provide objective evidence of an effective supplier management process. By tracking and analyzing these findings over time, companies can determine the effectiveness of their supplier management program.
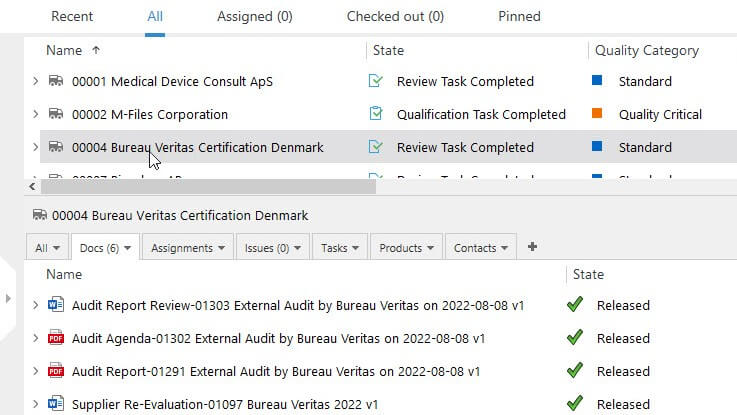
With SimplerQMS supplier management software, it is easy to manage all quality processes related to your suppliers in one system. The software offers pre-configured workflows based on Life Science requirements, allowing for streamlined supplier qualification, performance evaluations, and monitoring.
You can automate supplier audit plans by setting up email notifications to remind relevant persons about audit dates, supplier requalification, and review, certificate expiration dates, etc.
Our software also enables you to create actions and assign specific personnel, handle SCAR reports, attach certificates, and take actions to verify certificate accuracy, among many other features.
9. Equipment Management
Ensuring proper maintenance, calibration, and validation of quality critical equipment in highly regulated life sciences industries is essential.
Properly managed equipment reduces the risk of errors, defects, and failures that can negatively impact product quality and customer satisfaction.
The effectiveness of equipment management can be evaluated by tracking if the equipment has been calibrated in a timely manner and if there are overdue tasks.
Also, monitoring calibration and periodic maintenance tasks can help ensure equipment is effective and performed accordingly.
Using equipment management software, such as SimplerQMS, Life Science companies can streamline their equipment control, creating calibration and maintenance plans.
You can also automate notifications for assignments, overview equipment, and maintenance status, and link relevant documentation such as manuals, work instructions, certificates, and so on.
The system provides a documented history to identify actions performed on a piece of equipment and the responsible person(s).
Benefits of Ensuring an Effective Quality Management System
The more effective a company’s QMS, the less time and cost resources are needed to manage processes and handle quality issues.
Furthermore, some benefits of an effective QMS include the following:
Ensure Compliance
An effective QMS operates according to its intended purpose and complies with the specified requirements efficiently and adequately.
Effective QMS helps companies comply with industry regulations, standards, and customer requirements. It also helps ensure that the products are safe for their intended use.
Companies conduct audits to assess the effectiveness of their processes to ensure their actions are consistent and in compliance with the applicable requirements.
Increase Efficiency
Effective QMS allows companies to streamline their quality processes and identify improvement areas. This makes for a faster and more efficient process, leading to increased productivity and improved communication and collaboration.
Improve Time and Resources Management
Improved effectiveness of QMS processes can reduce the time and resources required to produce safe and reliable products by further streamlining the resource management process.
Moreover, with an eQMS in place, manual paperwork is reduced, allowing employees to focus their time and efforts on more value-adding activities, such as product development and quality improvement activities.
Reduce Costs
By streamlining quality processes, Life Science companies can significantly reduce their overall costs while improving quality.
Also, an effective production process can lead to fewer defective products, reducing overall manufacturing costs.
Streamline Risk Mitigation
Quality management systems allow companies to identify potential risks and assess the severity level. Moreover, companies must employ efforts to mitigate risks toward the end user.
The quality and safety of products are ensured through processes in compliance with the applicable requirements. With well-documented processes and procedures, it is easier and fast to detect potential quality issues.
Increase End User’s Confidence
An effective QMS helps companies comply with Life Science requirements applicable to them. This allows for placing safe, reliable, and effective products in the market.
Patients know they are getting a safe and reliable product, which can help build customer confidence in a company’s product.
Improve Your Quality Management System by Using QMS Software
Paper-based and hybrid QMS can be used to achieve compliance with regulatory and customer requirements.
However, Life Science companies can be more effective by using quality management software solutions.
Quality management software, like SimplerQMS, is designed for Life Science companies to help manage their quality process. The software helps maintain effective QMS processes with periodic checks, scheduled tasks, automatic notifications, and many other capabilities.
SimplerQMS helps handle quality management processes, such as document control, change management, training, audit management, CAPA management, and many other processes.
When compared to a manual QMS, some of the main benefits of an eQMS include the following:
- Decrease in human error
- Reduction in manual paperwork
- Increased efficiency
- Cost savings
- Easier compliance management
- Improved communication within the company
- Improved data integrity
- Secure access and sharing of documents
Despite the benefits which may already be apparent to you, comprehending the time and cost saved is essential. This helps to determine the decision of allocating resources towards implementing an eQMS.
To uncover the economic value an eQMS solution can bring to your company, you can download our free eQMS business case template.
The template allows you to compare your current QMS with a solution like SimplerQMS. It also includes a pre-made PowerPoint presentation that you can use to highlight your findings and explain the benefits to peers.
Final Thoughts
Regularly, the QMS is evaluated by top management in management reviews.
This helps check the system’s effectiveness and adequacy while defining the next quality objectives and implementing changes to processes and other elements of the QMS if necessary.
Modern electronic quality management systems also streamline and automate quality processes to improve effectiveness.
Implementing a comprehensive eQMS, like SimplerQMS, supports Life Science companies in increasing process effectiveness while reducing costs, saving time, and mitigating risks.
Using the SimplerQMS solution, you can streamline your quality management processes and make compliance easier. We offer a state-of-the-art document management system to formalize quality documentation.
Moreover, all our QMS modules are interlinked to provide a seamless workflow, such as document management, change control, employee training, complaints, CAPA management, audit management, and much more.
If you are interested in learning more, book a demo with one of our SimplerQMS software experts and join us on a journey toward continuous improvement in quality management.