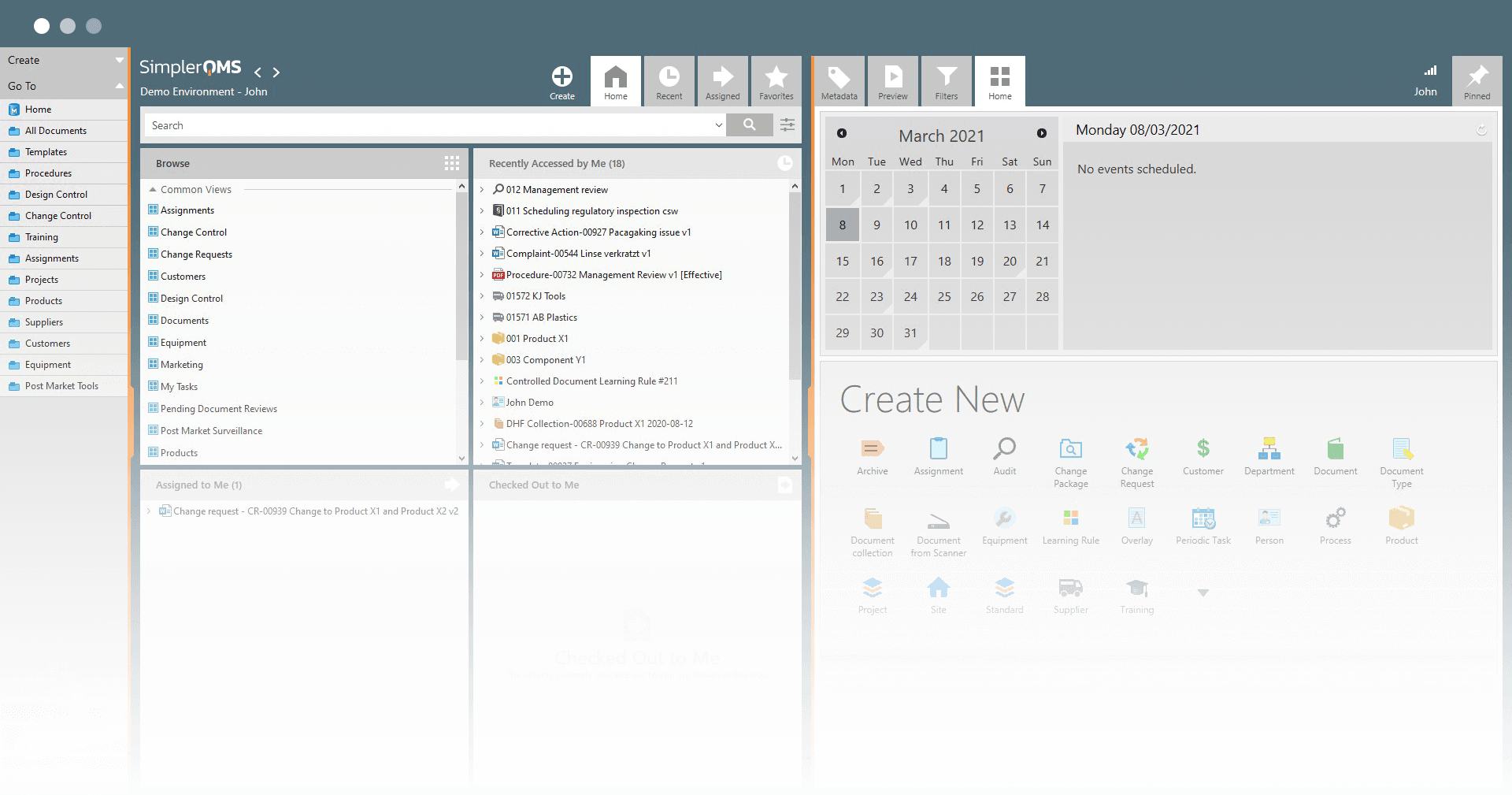
Life Science Quality Management Software
Comply with ISO, FDA, and GxP regulations and be audit-ready with our cloud-based quality management system.
Learn why life science organizations around the world trust SimplerQMS and achieve these results:
Full ISO, FDA, GxP, GMP compliance in audits
50% time reduction on change management
Increased efficiency and reduction in human error























Ready to Use
Automated Workflows
Electronic Signatures
Pre-Validated eQMS
For Life Science Organizations
Fully Compliant
ISO 13485:2016 Certified Vendor
MDR and IVDR Ready
ISO, FDA, GMP, GxP Compliant
GAMP5 Pre-Validated
Simple to Use
Easy User Interface
Integrated with Microsoft Office
Automated Document Upload
Works on Any Device
Out-of-the-Box Regulatory Compliance
We provide an audit trail, versioning, and eSignatures, which are ISO, GxP, MDR, and FDA Compliant.
SimplerQMS is pre-validated QMS software and is ready for audits and inspections. Furthermore, we ensure continuous re-validation of the system.
100% Transparent Pricing
With SimplerQMS you get everything for one subscription price, depending on the number of licenses.
There are NO implementation fees, extra charges for hosting, system validation, user training, ongoing support, or additional modules. It’s that simple.
One Platform That Connects All Quality Processes
Our quality management software integrates all your quality processes, and stores data in centralized, cloud storage, allowing you to manage risks, audits, suppliers, etc., in a single system.
From document control, with time-stamped audit trails and the FDA 21 CFR Part 11 compliant electronic signatures, to CAPAs or any other quality event, with automated assigning of tasks and notifications, SimplerQMS helps you work more efficiently and maintain compliance with ISO, FDA, and GxP regulations.
Document Control
Training Management
Risk Management
Design Control
Change Management
Supplier Management
CAPA Management
And much more…
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
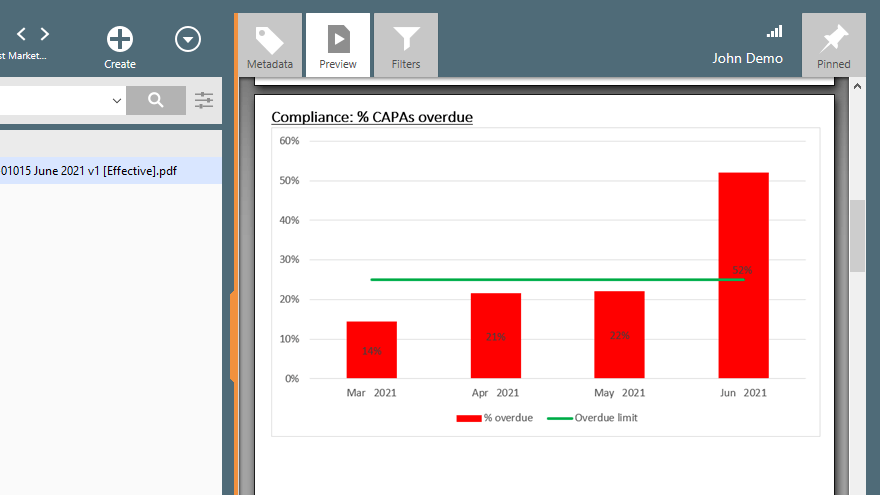
Pass audit more easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain high level of traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
Ready to learn more?
To learn how you can make the most of QMS software by SimplerQMS, request a free, personalized demo.