Life Science Quality Management Software
Automate your processes in a fully-validated Life Science QMS software with document control, change control, training, CAPAs, and other QMS modules in one package.
























All-In-One Solution
All eQMS Modules
Implementation
Support and Training
Fast Implementation
5-6 Weeks
Fully-Validated
Ready-To-Use Solution
Regulatory Compliance
Full ISO, FDA, GxP, GMP Compliance
Audit-Ready
Fully-Validated
Trusted Technology
Microsoft Cloud
5000+ Customers
20+ Years on the Market
Explore SimplerQMS Modules
Click play and find out how SimplerQMS manages the following eQMS modules in real time.
We seamlessly integrate with familiar Microsoft Office applications – Word, Excel, Outlook, PowerPoint. Work on your documents in Microsoft Office and store them with one click in SimplerQMS Cloud.
With SimplerQMS you can automate your change control processes. Create change requests, assign them to specific documents and delegate new assignments with automatic e-mail notifications.
You can assign training activities to specific employees and easily see an overview of the learning progress. The system also automatically reminds you when the training needs to be redone.
See SimplerQMS in Action
Take a look at some of the major features of the SimplerQMS software. The three major DEMO videos can be seen down below.
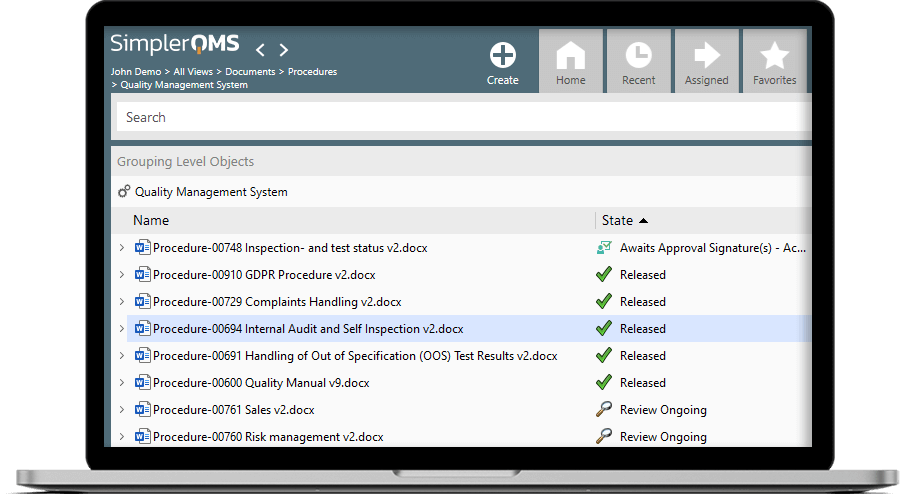
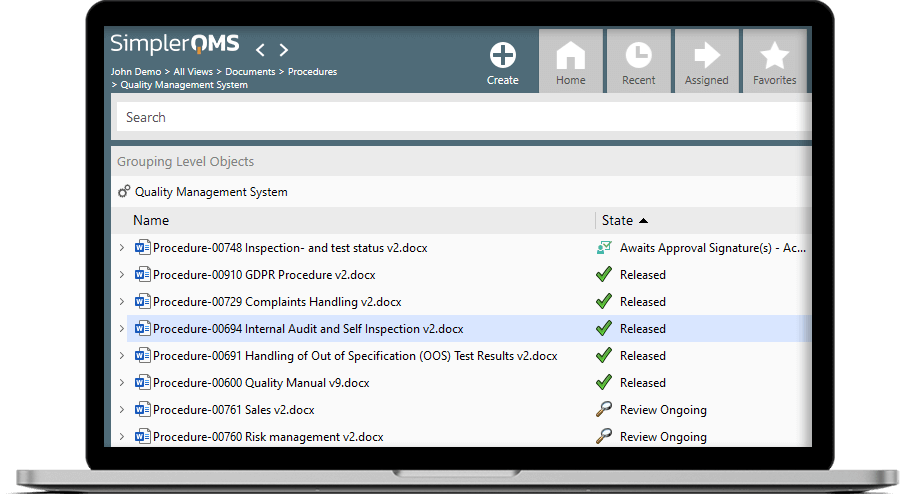
Document Control
We seamlessly integrate with familiar Microsoft Office applications – Word, Excel, Outlook, PowerPoint. Work on your documents in Microsoft Office and store them with one click in SimplerQMS Cloud.
Change Management
With SimplerQMS you can automate your change control processes. Create change requests, assign them to specific documents and delegate new assignments with automatic e-mail notifications.
Training Management
You can assign training activities to specific employees and easily see an overview of the learning progress. The system also automatically reminds you when the training needs to be redone.
Full Regulatory Compliance
SimplerQMS is fully validated according to GAMP5 and complies with FDA 21 CFR Part 11 and EU GMP Annex 11.
The system supports compliance with various Life Science requirements, such as GxP, ISO 13485:2016, FDA 21 CFR Part 210, 211, 820, ICH Q10, EU MDR and IVDR, ISO 9001:2015, and others.
Our customers have completed more than a hundred successful audits and inspections using SimplerQMS.
Avoid Time-Consuming Validation Processes
SimplerQMS is fully validated according to ISPE GAMP5 and is re-validated upon the creation of a new version or upon applying standard updates.
We do all the software validation for you – there are no additional expenses, resources, or time commitments to software validation on your part.
Have peace of mind with a system that complies with requirements regarding validation of systems used for QMS such as 21 CFR Part 11, and 820, EU GMP Annex 11, and ISO 13485:2016.
What kind of validation documentation and evidence do we provide?
- Validation Procedure
- Validation Plan
- Validation Report
- IQ, OQ and PQ Documentation
Recommended Reading: QMS Software Validation: When Is It Needed?
Designed For Life Sciences
Medical Device & MedTech
Ensure compliance with medical device regulation standards and simplify your quality processes.
Pharmaceutical & BioTech
Optimize workflows and reduce human error with automated quality management processes.
Clinical & Medical Laboratories
Ensure regulatory compliance and efficacy in your laboratory’s operations.
Trusted by Companies Around the World

Cortex Technology
Cortex Technology decreased the time spent on change management by 50% and increased task ownership.

Cortrium
Cortrium recorded major productivity boosts and decreased the number of human errors.

Reapplix
Reapplix team streamlined its quality processes and impress auditors during their first digital audit.
Without SimplerQMS
- Time-Consuming Processes
- Risk of Non-Compliance
- High Chance of Human Error
- Limited Documentation Access
- Risk of Non-Validated System
With SimplerQMS
- Efficient Processes
- Easier Compliance
- Reduced Human Error
- Securely Sign Documents Anywhere
- Audit-Ready and Fully-Validated
What Customers Achieve With SimplerQMS
Utilize Proven Technology
Our QMS for life-sciences is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass Audits Easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain a High Level of Traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Global Offices
With offices across 3 continents, we have the capability to offer truly global service and support.
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.
